Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior
- PMID: 24643294
- PMCID: PMC3958333
- DOI: 10.1371/journal.pbio.1001810
Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior
Abstract
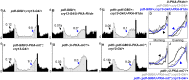
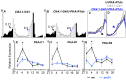
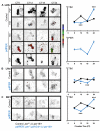
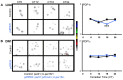
Molecular circadian clocks are interconnected via neural networks. In Drosophila, PIGMENT-DISPERSING FACTOR (PDF) acts as a master network regulator with dual functions in synchronizing molecular oscillations between disparate PDF(+) and PDF(-) circadian pacemaker neurons and controlling pacemaker neuron output. Yet the mechanisms by which PDF functions are not clear. We demonstrate that genetic inhibition of protein kinase A (PKA) in PDF(-) clock neurons can phenocopy PDF mutants while activated PKA can partially rescue PDF receptor mutants. PKA subunit transcripts are also under clock control in non-PDF DN1p neurons. To address the core clock target of PDF, we rescued per in PDF neurons of arrhythmic per⁰¹ mutants. PDF neuron rescue induced high amplitude rhythms in the clock component TIMELESS (TIM) in per-less DN1p neurons. Complete loss of PDF or PKA inhibition also results in reduced TIM levels in non-PDF neurons of per⁰¹ flies. To address how PDF impacts pacemaker neuron output, we focally applied PDF to DN1p neurons and found that it acutely depolarizes and increases firing rates of DN1p neurons. Surprisingly, these effects are reduced in the presence of an adenylate cyclase inhibitor, yet persist in the presence of PKA inhibition. We have provided evidence for a signaling mechanism (PKA) and a molecular target (TIM) by which PDF resets and synchronizes clocks and demonstrates an acute direct excitatory effect of PDF on target neurons to control neuronal output. The identification of TIM as a target of PDF signaling suggests it is a multimodal integrator of cell autonomous clock, environmental light, and neural network signaling. Moreover, these data reveal a bifurcation of PKA-dependent clock effects and PKA-independent output effects. Taken together, our results provide a molecular and cellular basis for the dual functions of PDF in clock resetting and pacemaker output.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The Drosophila Receptor Protein Tyrosine Phosphatase LAR Is Required for Development of Circadian Pacemaker Neuron Processes That Support Rhythmic Activity in Constant Darkness But Not during Light/Dark Cycles.J Neurosci. 2016 Mar 30;36(13):3860-70. doi: 10.1523/JNEUROSCI.4523-15.2016. J Neurosci. 2016. PMID: 27030770 Free PMC article.
-
PDF and cAMP enhance PER stability in Drosophila clock neurons.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):E1284-90. doi: 10.1073/pnas.1402562111. Epub 2014 Mar 18. Proc Natl Acad Sci U S A. 2014. PMID: 24707054 Free PMC article.
-
Drosophila CrebB is a Substrate of the Nonsense-Mediated mRNA Decay Pathway that Sustains Circadian Behaviors.Mol Cells. 2019 Apr 30;42(4):301-312. doi: 10.14348/molcells.2019.2451. Mol Cells. 2019. PMID: 31091556 Free PMC article.
-
Downloading central clock information in Drosophila.Mol Neurobiol. 2002 Oct-Dec;26(2-3):217-33. doi: 10.1385/MN:26:2-3:217. Mol Neurobiol. 2002. PMID: 12428757 Review.
-
Circadian clock genes in Drosophila: recent developments.Indian J Exp Biol. 2003 Aug;41(8):797-804. Indian J Exp Biol. 2003. PMID: 15248475 Review.
Cited by
-
Integration of Circadian Clock Information in the Drosophila Circadian Neuronal Network.J Biol Rhythms. 2021 Jun;36(3):203-220. doi: 10.1177/0748730421993953. Epub 2021 Mar 1. J Biol Rhythms. 2021. PMID: 33641476 Free PMC article. Review.
-
A Symphony of Signals: Intercellular and Intracellular Signaling Mechanisms Underlying Circadian Timekeeping in Mice and Flies.Int J Mol Sci. 2019 May 13;20(9):2363. doi: 10.3390/ijms20092363. Int J Mol Sci. 2019. PMID: 31086044 Free PMC article. Review.
-
High-Frequency Neuronal Bursting is Essential for Circadian and Sleep Behaviors in Drosophila.J Neurosci. 2021 Jan 27;41(4):689-710. doi: 10.1523/JNEUROSCI.2322-20.2020. Epub 2020 Dec 1. J Neurosci. 2021. PMID: 33262246 Free PMC article.
-
A Distinct Visual Pathway Mediates High-Intensity Light Adaptation of the Circadian Clock in Drosophila.J Neurosci. 2019 Feb 27;39(9):1621-1630. doi: 10.1523/JNEUROSCI.1497-18.2018. Epub 2019 Jan 3. J Neurosci. 2019. PMID: 30606757 Free PMC article.
-
Under warm ambient conditions, Drosophila melanogaster suppresses nighttime activity via the neuropeptide pigment dispersing factor.Genes Brain Behav. 2022 Apr;21(4):e12802. doi: 10.1111/gbb.12802. Epub 2022 Mar 14. Genes Brain Behav. 2022. PMID: 35285135 Free PMC article.
References
-
- Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, et al. (2003) vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112: 329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

