Purification of an IgA Monoclonal Antibody Specific for the Acr Protein of Mycobacterium tuberculosis by Immunoaffinity Chromatography
- PMID: 24643305
- PMCID: PMC3957348
Purification of an IgA Monoclonal Antibody Specific for the Acr Protein of Mycobacterium tuberculosis by Immunoaffinity Chromatography
Abstract
Background: A monoclonal antibody (mAb) of the IgA isotype, designated TBA61, is specific for the Acr protein of Mycobacterium tuberculosis (MTB). TBA61 has been used in studies exploring protection against tuberculosis (TB), and its efficacy has been proven using different challenge models. To purify the mouse IgA isotype, a combination of methods, such as globulin precipitation, ion exchange, and gel filtration, is usually required to achieve a satisfactory degree of purity.
Methods: To minimise the number of chromatographic steps, we proposed to employ immunoaffinity chromatography using the Acr protein of MTB as a specific ligand for this mAb. For this purpose, the HspX gene was cloned and expressed in Escherichia coli, and recombinant Acr (rAcr) was coupled to a cyanogen bromide-activated Sepharose 4B matrix, which was used to purify TBA61 mAb from ascites produced in mice in a single step.
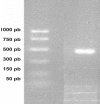
Results: The recovery from the purification procedure was 1.46 mg per mL of ascites. Analysis by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot showed a high purity. The purified mAb retained its reactivity against the Acr protein based on enzyme-linked immunosorbent assay (ELISA) and western blot.
Conclusion: The purification method used is rapid, simple, and specific and can be easily scaled up.
Keywords: Acr protein; IgA; Mycobacterium tuberculosis; affinity; chromatography; monoclonal antibody.
Figures



References
-
- World Health Organization . Geneva (CH): World Health Organization; 2013. [cited 2013]. Tuberculosis. [Internet] doi: http://www.who.int/mediacentre/factsheets/fs104/en/
-
- World Health Organization . Geneva (CH): WHO report; 2008. Global Tuberculosis Control 2008: Surveillance, Planning, Financing; pp. 1–37.
-
- Center for Disease Control and Prevention (CDC) Emergence of Mycobacterium tuberculosis with extensive resistance to secondline drugs worldwide, 2002–2004. MMWR Morb Mort Wkly Rep. 2006;55(11):301–305. - PubMed
-
- Anonymous The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the advisory council for the elimination of tuberculosis and the advisory committee on immunization practices. MMWR Recomm Rep. 1996;45(RR-4):1–18. - PubMed
-
- Falero-Diaz G, Challacombe S, Rahman D, Mistry M, Douce G, Dougan G, et al. Transmission of IgA and IgG monoclonal antibodies to mucosal fluids following intranasal or parenteral delivery. Int Arch Allergy Immunol. 2000;122(2):143–150. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
