Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles
- PMID: 24644038
- PMCID: PMC4362695
- DOI: 10.1002/ana.24145
Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles
Abstract
Objective: High level of homocysteine (Hcy) is a recognized risk factor for developing Alzheimer disease (AD). However, the mechanisms involved are unknown. Previously, it was shown that high Hcy increases brain β-amyloid (Aβ) levels in amyloid precursor protein transgenic mice, but no data are available on the effect that it may have on the other main pathologic features of AD such as tau.
Methods: 3xTg mice with diet-induced high Hcy were compared with mice having normal Hcy. Neuronal cells were incubated with and without Hcy.
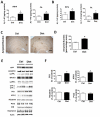
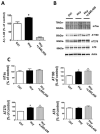
Results: Diet-induced high Hcy resulted in an exacerbation of the entire AD-like phenotype of the 3xTg mice. In particular, we found that compared with controls, mice with high Hcy developed significant memory and learning deficits, and had elevated Aβ levels and deposition, which was mediated by an activation of the γ-secretase pathway. In addition, the same mice had a significant increase in the insoluble fraction of tau and its phosphorylation at specific epitopes, which was mediated by the cdk5 pathway. In vitro studies confirmed these observations and provided evidence that the effects of Hcy on Aβ and tau are independent from each other.
Interpretation: Taken together, our findings demonstrate that a dietary condition that leads to an elevation of Hcy levels results in an exacerbation of all 3 major pathological features of the AD phenotype: memory deficits, and Aβ and tau neuropathology. They support the concept that this dietary lifestyle can act as a risk factor and actively contribute to the development of the disease.
© 2014 American Neurological Association.
Figures





References
-
- Morris MS. Homocysteine and Alzheimer’s disease. Lancet Neurol. 2003;2:425–428. - PubMed
-
- Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. - PubMed
-
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. - PubMed
-
- Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer’s disease: a systematic review. Arch Gerontol Geriatr. 2009;48:425–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

