Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience
- PMID: 24644078
- PMCID: PMC4265196
- DOI: 10.1002/pds.3593
Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience
Abstract
Purpose: To summarise post-licensure safety surveillance over more than 4 years of routine use of the human papillomavirus-16/18-AS04-adjuvanted vaccine (HPV-16/18 vaccine: Cervarix®, GlaxoSmithKline, Belgium).
Methods: We describe global post-licensure passive surveillance data based on routine pharmacovigilance from 18 May 2007 until 17 November 2011 and enhanced surveillance implemented during the 2-year national immunisation programme in the UK (school years 2008-2010).
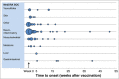
Results: Spontaneous reports from countries worldwide showed a similar pattern for the most frequently reported adverse events after HPV-16/18 vaccination. No patterns or trends were observed for potential immune-mediated diseases after vaccination. Observed incidences of Bell's palsy and confirmed Guillain-Barré syndrome were within the expected range in the general population. Outcomes of pregnancy in women who were inadvertently exposed to HPV-16/18 vaccine during pregnancy, were in line with published reports for similar populations. Enhanced surveillance of adverse events in the UK triggered a review of cases of anaphylaxis, angioedema and syncope reports, leading to an update to the prescribing information.
Conclusion: Collaborative partnerships between industry and national regulatory agencies facilitated rapid notification and transfer of safety information, allowing for rapid responses in the event of a safety signal of adverse event of concern. More than 4 years of post-licensure experience may provide confidence to providers and the public about the safety profile of HPV-16/18 vaccine in routine use. The safety profile appears to be consistent with pre-licensure data reporting that HPV-16/18 vaccine has an acceptable benefit-risk profile in adolescent girls and women.
Keywords: AS04; adverse drug reactions; human papillomavirus vaccine; pharmacoepidemiology; post-licensure surveillance; potential immune-mediated diseases; pregnancy.
© 2014 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Figures
References
-
- Macartney KK, Chiu C, Georgousakis M, Brotherton JML. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;36:393–412. - PubMed
-
- Medical Dictionary for Regulatory Activities Maintenance and Support Services Organization. 2012. http://www.meddramsso.com/ (accessed 21 November 2012)
-
- Report of CIOMS Working Group IV. Benefit–risk balance for marketed drugs: evaluating safety signals. 1998. http://www.cioms.ch/publications/g4-benefit-risk.pdf (accessed 21 November 2012)
-
- Verstraeten T, Descamps D, David M-P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26:6630–6638. - PubMed
-
- Cervarix Summary of Product Characteristics. 2012. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed 21 November 2012)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

