Cleavage within Reelin repeat 3 regulates the duration and range of the signaling activity of Reelin protein
- PMID: 24644294
- PMCID: PMC4007479
- DOI: 10.1074/jbc.M113.536326
Cleavage within Reelin repeat 3 regulates the duration and range of the signaling activity of Reelin protein
Abstract
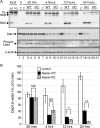
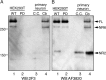
Reelin is a secreted glycoprotein that plays essential roles in the brain. Reelin is specifically cleaved at two distinct sites, called N-t and C-t, with the former being the major one. N-t cleavage can occur both in the extracellular space and in the endosomes, although the physiological importance of endosomal N-t cleavage has not been investigated. In this study, we first determined the exact N-t cleavage site catalyzed by a protease secreted by cerebral cortical neurons. Cleavage occurred between Pro-1244 and Ala-1245 within Reelin repeat 3. A Reelin mutant in which Pro-1244 was replaced with aspartate (Reelin-PD) was resistant to a protease secreted by cultured cerebral cortical neurons, and its biological activity stayed active longer than that of wild-type Reelin. Interestingly, Reelin-PD remained in the intracellular compartments longer than wild-type Reelin and persistently activated downstream signaling. Therefore, N-t cleavage of Reelin is required for halting the signaling machinery in the extracellular space as well as within endosomes of target neurons. We established a monoclonal antibody specific to uncleaved Reelin protein and found that it is localized in the vicinity of Reelin-producing cells, whereas the N-terminal fragment diffuses, or is transported, to distant regions. These data demonstrate that N-t cleavage of Reelin plays critical roles in regulating the duration and range of Reelin functions both in the extracellular milieu and in the intracellular compartments.
Keywords: ADAM ADAMTS; Brain; Dab1; Endosomes; Neurons; Protein Degradation; Reelin; Signal Transduction.
Figures





References
-
- Tissir F., Goffinet A. M. (2003) Reelin and brain development. Nat. Rev. Neurosci. 4, 496–505 - PubMed
-
- Tiberi L., Vanderhaeghen P., van den Ameele J. (2012) Cortical neurogenesis and morphogens: diversity of cues, sources and functions. Curr. Opin. Cell Biol. 24, 269–276 - PubMed
-
- Park H., Poo M. M. (2013) Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 - PubMed
-
- Baker K. A., Moore S. W., Jarjour A. A., Kennedy T. E. (2006) When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr. Opin. Neurobiol. 16, 529–534 - PubMed
-
- Rogers K. W., Schier A. F. (2011) Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27, 377–407 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

