Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise
- PMID: 24644429
- PMCID: PMC3957089
- DOI: 10.7150/ijbs.7972
Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise
Abstract
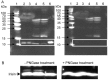
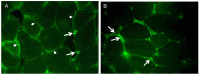
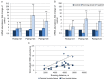
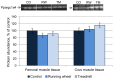
Recent findings regarding the response of fibronectin type III domain-containing protein 5 (Fndc5) and irisin to exercise are partly controversial. While the 25 kDa form of Fndc5 can be observed in muscle and serum of different species, the ~12 kDa irisin band was not detectable up to now. The present study aimed to clarify whether irisin exists in its theoretical size of ~12 kDa in mice and if it is affected by exercise. Male mice were randomly assigned to a sedentary control group (CO), a group with free access to running wheels (RW), and a treadmill group (TM). Blood and leg muscles were collected to investigate the regulatory cascade including peroxisome proliferator-activated receptor gamma co-activator 1-alpha (Ppargc1a) and Fndc5. In western blot analysis, antibodies were used capable of differentiation between full-length Fndc5 and irisin. This enabled us to demonstrate that irisin exists in muscle and serum of mice independent of exercise and that it is increased immediately after acute exercise. Different transcripts of Ppargc1a mRNA, but not Fndc5 mRNA, were up-regulated in the TM group. Furthermore, neither Fndc5 (25 kDa) nor Ppargc1a protein was elevated in muscle tissue. The Ppargc1a-Fndc5/irisin pathway did not clearly respond to mild exercise in the RW group. Our results provide evidence for the existence of irisin and for its immediate response to acute exercise in mice.
Keywords: Fndc5; Ppargc1a.; endurance exercise; irisin; myokine.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C, Viguerie N. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.158. - PubMed
-
- Boström P, Wu J, Jedrychowski MP Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. - PMC - PubMed
-
- Brenmoehl J, Walz C, Renne U, Ponsuksili S, Wolf C, Langhammer M, Schwerin M, Hoeflich A. Metabolic adaptations in the liver of born long-distance running mice. Med Sci Sports Exerc. 2013;45:841–850. - PubMed

