Role for tissue-dependent methylation differences in the expression of FOXE1 in nontumoral thyroid glands
- PMID: 24646064
- PMCID: PMC5050036
- DOI: 10.1210/jc.2013-4414
Role for tissue-dependent methylation differences in the expression of FOXE1 in nontumoral thyroid glands
Abstract
Background: Discordance of monozygotic twins for thyroid dysgenesis suggests that epigenetic mechanisms may underlie defects in thyroid gland development. This prompted us to evaluate whether differentially methylated regions (DMRs) can be found between human thyroids (either eutopic or ectopic) and matched leukocytes.
Methods: To compare the genome-wide methylation profile of thyroids and leukocytes, immunoprecipitated methylated DNA was interrogated on human promoter plus CpG island tiling arrays. In addition, the methylation profile of the human FOXE1, PAX8, and NKX2.1 promoter was examined using bisulfite sequencing. Finally, the functional impact of CpG methylation of the promoter on FOXE1 expression was assessed with luciferase assays.
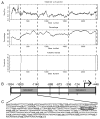
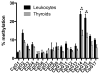
Results: Genome-wide methylation profiling and bisulfite sequencing of CpG islands of PAX8 and NKX2.1 promoters revealed no DMR between thyroid and leukocytes. However, bisulfite sequencing revealed that the methylation level of two consecutive CpG dinucleotides (CpG14 and CpG15, which were not covered by the genome-wide array) in one CpG island of the FOXE1 promoter (-1600 to -1140 from the transcription start site) is significantly higher in leukocytes than in eutopic or ectopic thyroid tissues, suggesting that methylation of this region may decrease FOXE1 gene expression. Indeed, luciferase activities were decreased when FOXE1 promoter constructs were methylated in vitro. Moreover, derepression of luciferase activity was observed when the methylation of CpG14 and CpG15 was prevented by mutations.
Conclusion: We report a tissue-dependent DMR in the FOXE1 promoter. This DMR contains two consecutive CpG dinucleotides, which are epigenetic modifiers of FOXE1 expression in nontumoral tissues.
Figures



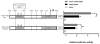
Similar articles
-
Reduced expression of FOXE1 in differentiated thyroid cancer, the contribution of CPG methylation, and their clinical relevance.Front Endocrinol (Lausanne). 2024 Nov 11;15:1454349. doi: 10.3389/fendo.2024.1454349. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39588344 Free PMC article.
-
Transcriptome, methylome and genomic variations analysis of ectopic thyroid glands.PLoS One. 2010 Oct 15;5(10):e13420. doi: 10.1371/journal.pone.0013420. PLoS One. 2010. PMID: 20976176 Free PMC article.
-
New insights into FoxE1 functions: identification of direct FoxE1 targets in thyroid cells.PLoS One. 2013 May 13;8(5):e62849. doi: 10.1371/journal.pone.0062849. Print 2013. PLoS One. 2013. PMID: 23675434 Free PMC article.
-
Regulation of Foxe1 by Thyrotropin and Transforming Growth Factor Beta Depends on the Interplay Between Thyroid-Specific, CREB and SMAD Transcription Factors.Thyroid. 2019 May;29(5):714-725. doi: 10.1089/thy.2018.0136. Epub 2019 Feb 13. Thyroid. 2019. PMID: 30652527
-
CpG island methylation profiling in human salivary gland adenoid cystic carcinoma.Cancer. 2011 Jul 1;117(13):2898-909. doi: 10.1002/cncr.25818. Epub 2011 Jan 11. Cancer. 2011. PMID: 21692051 Free PMC article.
Cited by
-
FOXE1 regulates migration and invasion in thyroid cancer cells and targets ZEB1.Endocr Relat Cancer. 2020 Mar;27(3):137-151. doi: 10.1530/ERC-19-0156. Endocr Relat Cancer. 2020. PMID: 31846430 Free PMC article.
-
Thyroid transcription factors in development, differentiation and disease.Nat Rev Endocrinol. 2015 Jan;11(1):29-42. doi: 10.1038/nrendo.2014.186. Epub 2014 Oct 28. Nat Rev Endocrinol. 2015. PMID: 25350068 Review.
-
Genetics of primary congenital hypothyroidism: three decades of discoveries and persisting etiological challenges.Eur Thyroid J. 2025 Mar 28;14(2):e240348. doi: 10.1530/ETJ-24-0348. Print 2025 Apr 1. Eur Thyroid J. 2025. PMID: 40100854 Free PMC article. Review.
-
Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer.Int J Mol Sci. 2024 Feb 6;25(4):1966. doi: 10.3390/ijms25041966. Int J Mol Sci. 2024. PMID: 38396644 Free PMC article.
-
DNA methylation at a nutritionally sensitive region of the PAX8 gene is associated with thyroid volume and function in Gambian children.Sci Adv. 2021 Nov 5;7(45):eabj1561. doi: 10.1126/sciadv.abj1561. Epub 2021 Nov 5. Sci Adv. 2021. PMID: 34739318 Free PMC article.
References
-
- Dathan N, Parlato R, Rosica A, De Felice M, Di Lauro R. Distribution of the titf2/foxe1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev Dyn. 2002;224:450–456. - PubMed
-
- De Felice M, Ovitt C, Biffali E, et al. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19:395–398. - PubMed
-
- Clifton-Bligh RJ, Wentworth JM, Heinz P, et al. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet. 1998;19:399–401. - PubMed
-
- Castanet M, Park SM, Smith A, et al. A novel loss-of-function mutation in TTF-2 is associated with congenital hypothyroidism, thyroid agenesis and cleft palate. Hum Mol Genet. 2002;11:2051–2059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

