Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma
- PMID: 24646471
- PMCID: PMC4023416
- DOI: 10.1182/blood-2014-01-550020
Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma
Abstract
We assessed the prognostic value of minimal residual disease (MRD) detection in multiple myeloma (MM) patients using a sequencing-based platform in bone marrow samples from 133 MM patients in at least very good partial response (VGPR) after front-line therapy. Deep sequencing was carried out in patients in whom a high-frequency myeloma clone was identified and MRD was assessed using the IGH-VDJH, IGH-DJH, and IGK assays. The results were contrasted with those of multiparametric flow cytometry (MFC) and allele-specific oligonucleotide polymerase chain reaction (ASO-PCR). The applicability of deep sequencing was 91%. Concordance between sequencing and MFC and ASO-PCR was 83% and 85%, respectively. Patients who were MRD(-) by sequencing had a significantly longer time to tumor progression (TTP) (median 80 vs 31 months; P < .0001) and overall survival (median not reached vs 81 months; P = .02), compared with patients who were MRD(+). When stratifying patients by different levels of MRD, the respective TTP medians were: MRD ≥10(-3) 27 months, MRD 10(-3) to 10(-5) 48 months, and MRD <10(-5) 80 months (P = .003 to .0001). Ninety-two percent of VGPR patients were MRD(+). In complete response patients, the TTP remained significantly longer for MRD(-) compared with MRD(+) patients (131 vs 35 months; P = .0009).
© 2014 by The American Society of Hematology.
Figures
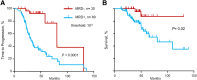

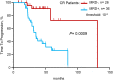
Comment in
-
Down to the bitter end.Blood. 2014 May 15;123(20):3061-2. doi: 10.1182/blood-2014-04-565317. Blood. 2014. PMID: 24832937
References
-
- Corso A, Nozza A, Lazzarino M, et al. Plateau phase in multiple myeloma: an end-point of conventional-dose chemotherapy. Haematologica. 1999;84(4):336–341. - PubMed
-
- Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists’ Collaborative Group. J Clin Oncol. 1998;16(12):3832–3842. - PubMed
-
- Barlogie B, Hall R, Zander A, Dicke K, Alexanian R. High-dose melphalan with autologous bone marrow transplantation for multiple myeloma. Blood. 1986;67(5):1298–1301. - PubMed
-
- Bladé J, Samson D, Reece D, et al. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115–1123. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

