Ca²⁺ signaling and regulation of fluid secretion in salivary gland acinar cells
- PMID: 24646566
- PMCID: PMC4059182
- DOI: 10.1016/j.ceca.2014.02.009
Ca²⁺ signaling and regulation of fluid secretion in salivary gland acinar cells
Abstract
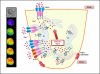
Neurotransmitter stimulation of plasma membrane receptors stimulates salivary gland fluid secretion via a complex process that is determined by coordinated temporal and spatial regulation of several Ca(2+) signaling processes as well as ion flux systems. Studies over the past four decades have demonstrated that Ca(2+) is a critical factor in the control of salivary gland function. Importantly, critical components of this process have now been identified, including plasma membrane receptors, calcium channels, and regulatory proteins. The key event in activation of fluid secretion is an increase in intracellular [Ca(2+)] ([Ca(2+)]i) triggered by IP3-induced release of Ca(2+) from ER via the IP3R. This increase regulates the ion fluxes required to drive vectorial fluid secretion. IP3Rs determine the site of initiation and the pattern of [Ca(2+)]i signal in the cell. However, Ca(2+) entry into the cell is required to sustain the elevation of [Ca(2+)]i and fluid secretion. This Ca(2+) influx pathway, store-operated calcium influx pathway (SOCE), has been studied in great detail and the regulatory mechanisms as well as key molecular components have now been identified. Orai1, TRPC1, and STIM1 are critical components of SOCE and among these, Ca(2+) entry via TRPC1 is a major determinant of fluid secretion. The receptor-evoked Ca(2+) signal in salivary gland acinar cells is unique in that it starts at the apical pole and then rapidly increases across the cell. The basis for the polarized Ca(2+) signal can be ascribed to the polarized arrangement of the Ca(2+) channels, transporters, and signaling proteins. Distinct localization of these proteins in the cell suggests compartmentalization of Ca(2+) signals during regulation of fluid secretion. This chapter will discuss new concepts and findings regarding the polarization and control of Ca(2+) signals in the regulation of fluid secretion.
Keywords: Calcium signaling; IP3R; Orai1; STIM1; Salivary fluid secretion; TRPC1.
Published by Elsevier Ltd.
Figures


References
-
- Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. - PubMed
-
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. - PubMed
-
- Mikoshiba K, Hisatsune C, Futatsugi A, Mizutani A, Nakamura T, Miyachi K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea. 2008;27(Suppl 1):S3–8. - PubMed
-
- Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

