Vaccines that stimulate T cell immunity to HIV-1: the next step
- PMID: 24646598
- PMCID: PMC4324504
- DOI: 10.1038/ni.2844
Vaccines that stimulate T cell immunity to HIV-1: the next step
Abstract
The search for a vaccine against human immunodeficiency virus type 1 (HIV-1) has many hurdles to overcome. Ideally, the stimulation of both broadly neutralizing antibodies and cell-mediated immune responses remains the best option, but no candidate in clinical trials at present has elicited such antibodies, and efficacy trials have not demonstrated any benefit for vaccines designed to stimulate immune responses of CD8(+) T cells. Findings obtained with the simian immunodeficiency virus (SIV) monkey model have provided new evidence that stimulating effective CD8(+) T cell immunity could provide protection, and in this Perspective we explore the path forward for optimizing such responses in humans.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
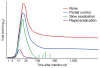
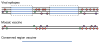
References
-
- Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

