An eight-year clinic experience with clozapine use in a Parkinson's disease clinic setting
- PMID: 24646688
- PMCID: PMC3960134
- DOI: 10.1371/journal.pone.0091545
An eight-year clinic experience with clozapine use in a Parkinson's disease clinic setting
Abstract
Background: To examine our eight year clinic-based experience in a Parkinson's disease expert clinical care center using clozapine as a treatment for refractory psychosis in Parkinson's disease (PD).
Methods: The study was a retrospective chart review which covered eight years of clozapine registry use. Statistical T-tests, chi-square, correlations and regression analysis were used to analyze treatment response for potential associations of age, disease duration, and Hoehn & Yahr (H&Y) score, and degree of response to clozapine therapy.
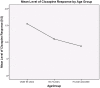
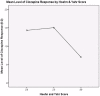
Results: There were 36 participants included in the analysis (32 PD, 4 parkinsonism-plus). The characteristics included 30.6% female, age 45-87 years (mean 68.3±10.15), disease duration of 17-240 months (mean 108.14±51.13) and H&Y score of 2 to 4 (mean 2.51±0.51). The overall retention rate on clozapine was 41% and the most common reasons for discontinuation were frequent blood testing (28%), nursing home (NH) placement (11%) and leucopenia (8%). Responses to clozapine across the cohort were: complete (33%), partial (33%), absent (16%), and unknown (16%). Age (r = -0.36, p<0.01) and H&Y score (r = -0.41, p<0.01) were shown to be related to response to clozapine therapy, but disease duration was not an associated factor (r = 0.21, p>0.05).
Conclusions: This single-center experience highlights the challenges associated with clozapine therapy in PD psychosis. Frequent blood testing remains a significant barrier for clozapine, even in patients with therapeutic benefit. Surprisingly, all patients admitted to a NH discontinued clozapine due to logistical issues of administration and monitoring within that setting. Consideration of the barriers to clozapine therapy will be important to its use and to its continued success in an outpatient setting.
Conflict of interest statement
Figures
References
-
- Morgante L, Epifanio A, Spina E, Zappia M, Di Rosa AE, et al. (2004) Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol 27 4: 153–156. - PubMed
-
- Merims D, Balas M, Peretz C, Shabtai H, Giladi N (2006) Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson's disease psychosis. Clin Neuropharmacol 29 6: 331–337. - PubMed
-
- Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J (1998) Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry 59 Suppl 3: 3–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical