A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients
- PMID: 24646861
- PMCID: PMC4150998
- DOI: 10.1038/ki.2014.58
A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients
Abstract
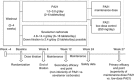
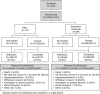
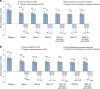
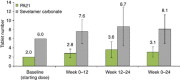
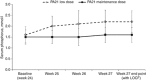
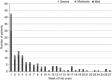
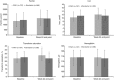
Efficacy of PA21 (sucroferric oxyhydroxide), a novel calcium-free polynuclear iron(III)-oxyhydroxide phosphate binder, was compared with that of sevelamer carbonate in an open-label, randomized, active-controlled phase III study. Seven hundred and seven hemo- and peritoneal dialysis patients with hyperphosphatemia received PA21 1.0-3.0 g per day and 348 received sevelamer 4.8-14.4 g per day for an 8-week dose titration, followed by 4 weeks without dose change, and then 12 weeks maintenance. Serum phosphorus reductions at week 12 were -0.71 mmol/l (PA21) and -0.79 mmol/l (sevelamer), demonstrating non-inferiority of, on average, three tablets of PA21 vs. eight of sevelamer. Efficacy was maintained to week 24. Non-adherence was 15.1% (PA21) vs. 21.3% (sevelamer). The percentage of patients that reported at least one treatment-emergent adverse event was 83.2% with PA21 and 76.1% with sevelamer. A higher proportion of patients withdrew owing to treatment-emergent adverse events with PA21 (15.7%) vs. sevelamer (6.6%). Mild, transient diarrhea, discolored feces, and hyperphosphatemia were more frequent with PA21; nausea and constipation were more frequent with sevelamer. After 24 weeks, 99 hemodialysis patients on PA21 were re-randomized into a 3-week superiority analysis of PA21 maintenance dose in 50 patients vs. low dose (250 mg per day (ineffective control)) in 49 patients. The PA21 maintenance dose was superior to the low dose in maintaining serum phosphorus control. Thus, PA21 was effective in lowering serum phosphorus in dialysis patients, with similar efficacy to sevelamer carbonate, a lower pill burden, and better adherence.
Figures
Comment in
-
Clinical trials. Less of the same--PA21 can reduce pill burden in patients with hyperphosphataemia.Nat Rev Nephrol. 2014 Jun;10(6):297. doi: 10.1038/nrneph.2014.62. Epub 2014 Apr 8. Nat Rev Nephrol. 2014. PMID: 24709845 No abstract available.
-
Novel phosphate binders: plus ça change, plus c'est la même chose.Kidney Int. 2014 Sep;86(3):471-4. doi: 10.1038/ki.2014.177. Kidney Int. 2014. PMID: 25168499
References
-
- Block GA. Control of serum phosphorus: implications for coronary artery calcification and calcific uremic arteriolopathy (calciphylaxis) Curr Opin Nephrol Hypertens. 2001;10:741–747. - PubMed
-
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. - PubMed
-
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. - PubMed
-
- Kanbay M, Goldsmith D, Akcay A, et al. Phosphate—the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009;27:220–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

