Unexpected link between an antibiotic, pannexin channels and apoptosis
- PMID: 24646995
- PMCID: PMC4078991
- DOI: 10.1038/nature13147
Unexpected link between an antibiotic, pannexin channels and apoptosis
Abstract
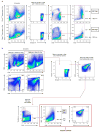
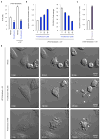
Plasma membrane pannexin 1 channels (PANX1) release nucleotide find-me signals from apoptotic cells to attract phagocytes. Here we show that the quinolone antibiotic trovafloxacin is a novel PANX1 inhibitor, by using a small-molecule screen. Although quinolones are widely used to treat bacterial infections, some quinolones have unexplained side effects, including deaths among children. PANX1 is a direct target of trovafloxacin at drug concentrations seen in human plasma, and its inhibition led to dysregulated fragmentation of apoptotic cells. Genetic loss of PANX1 phenocopied trovafloxacin effects, revealing a non-redundant role for pannexin channels in regulating cellular disassembly during apoptosis. Increase in drug-resistant bacteria worldwide and the dearth of new antibiotics is a major human health challenge. Comparing different quinolone antibiotics suggests that certain structural features may contribute to PANX1 blockade. These data identify a novel linkage between an antibiotic, pannexin channels and cellular integrity, and suggest that re-engineering certain quinolones might help develop newer antibacterials.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

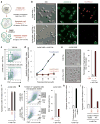


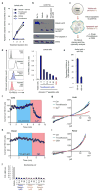

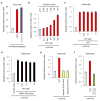

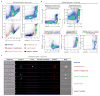



Comment in
-
Cell biology: The disassembly of death.Nature. 2014 Mar 20;507(7492):312-3. doi: 10.1038/nature13213. Epub 2014 Mar 12. Nature. 2014. PMID: 24646991 No abstract available.
References
-
- Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

