Development of a physiologically-based pharmacokinetic model of the rat central nervous system
- PMID: 24647103
- PMCID: PMC3978528
- DOI: 10.3390/pharmaceutics6010097
Development of a physiologically-based pharmacokinetic model of the rat central nervous system
Abstract
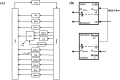
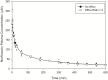
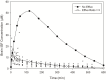
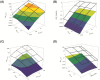
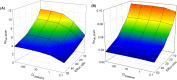
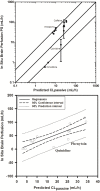
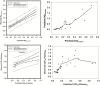
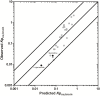
Central nervous system (CNS) drug disposition is dictated by a drug's physicochemical properties and its ability to permeate physiological barriers. The blood-brain barrier (BBB), blood-cerebrospinal fluid barrier and centrally located drug transporter proteins influence drug disposition within the central nervous system. Attainment of adequate brain-to-plasma and cerebrospinal fluid-to-plasma partitioning is important in determining the efficacy of centrally acting therapeutics. We have developed a physiologically-based pharmacokinetic model of the rat CNS which incorporates brain interstitial fluid (ISF), choroidal epithelial and total cerebrospinal fluid (CSF) compartments and accurately predicts CNS pharmacokinetics. The model yielded reasonable predictions of unbound brain-to-plasma partition ratio (Kpuu,brain) and CSF:plasma ratio (CSF:Plasmau) using a series of in vitro permeability and unbound fraction parameters. When using in vitro permeability data obtained from L-mdr1a cells to estimate rat in vivo permeability, the model successfully predicted, to within 4-fold, Kpuu,brain and CSF:Plasmau for 81.5% of compounds simulated. The model presented allows for simultaneous simulation and analysis of both brain biophase and CSF to accurately predict CNS pharmacokinetics from preclinical drug parameters routinely available during discovery and development pathways.
Figures



Similar articles
-
Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics.Adv Drug Deliv Rev. 2004 Oct 14;56(12):1825-57. doi: 10.1016/j.addr.2004.07.011. Adv Drug Deliv Rev. 2004. PMID: 15381336 Review.
-
Lumbar cerebrospinal fluid-to-brain extracellular fluid surrogacy is context-specific: insights from LeiCNS-PK3.0 simulations.J Pharmacokinet Pharmacodyn. 2021 Oct;48(5):725-741. doi: 10.1007/s10928-021-09768-7. Epub 2021 Jun 17. J Pharmacokinet Pharmacodyn. 2021. PMID: 34142308 Free PMC article.
-
[Kinetic analysis of the disposition of hydrophilic drugs in the central nervous system (CNS): prediction of the CNS disposition from the transport properties in the blood-brain and blood-cerebrospinal fluid barriers].Yakugaku Zasshi. 1994 Dec;114(12):950-71. doi: 10.1248/yakushi1947.114.12_950. Yakugaku Zasshi. 1994. PMID: 7869236 Review. Japanese.
-
CSF as a surrogate for assessing CNS exposure: an industrial perspective.Curr Drug Metab. 2008 Jan;9(1):46-59. doi: 10.2174/138920008783331077. Curr Drug Metab. 2008. PMID: 18220571 Review.
-
Translational CNS Steady-State Drug Disposition Model in Rats, Monkeys, and Humans for Quantitative Prediction of Brain-to-Plasma and Cerebrospinal Fluid-to-Plasma Unbound Concentration Ratios.AAPS J. 2021 Jun 3;23(4):81. doi: 10.1208/s12248-021-00609-6. AAPS J. 2021. PMID: 34085128 Free PMC article.
Cited by
-
Acetylcholinesterase Inhibition in Rats and Humans Following Acute Fenitrothion Exposure Predicted by Physiologically Based Kinetic Modeling-Facilitated Quantitative In Vitro to In Vivo Extrapolation.Environ Sci Technol. 2023 Dec 12;57(49):20521-20531. doi: 10.1021/acs.est.3c07077. Epub 2023 Nov 26. Environ Sci Technol. 2023. PMID: 38008925 Free PMC article.
-
Microdialysis: the Key to Physiologically Based Model Prediction of Human CNS Target Site Concentrations.AAPS J. 2017 Jul;19(4):891-909. doi: 10.1208/s12248-017-0050-3. Epub 2017 Mar 9. AAPS J. 2017. PMID: 28281195 Review.
-
Using the LeiCNS-PK3.0 Physiologically-Based Pharmacokinetic Model to Predict Brain Extracellular Fluid Pharmacokinetics in Mice.Pharm Res. 2023 Nov;40(11):2555-2566. doi: 10.1007/s11095-023-03554-5. Epub 2023 Jul 13. Pharm Res. 2023. PMID: 37442882 Free PMC article.
-
Emerging therapeutics and evolving assessment criteria for intracranial metastases in patients with oncogene-driven non-small-cell lung cancer.Nat Rev Clin Oncol. 2023 Oct;20(10):716-732. doi: 10.1038/s41571-023-00808-4. Epub 2023 Aug 17. Nat Rev Clin Oncol. 2023. PMID: 37592034 Free PMC article. Review.
-
Semi-Mechanistic Population Pharmacokinetic Modeling of L-Histidine Disposition and Brain Uptake in Wildtype and Pht1 Null Mice.Pharm Res. 2018 Jan 5;35(1):19. doi: 10.1007/s11095-017-2322-0. Pharm Res. 2018. PMID: 29305823 Free PMC article.
References
-
- Brodie B.B., Kurz H., Schanker L.S. The importance of dissociaton constant and lipid-solubility in influencing the passage of drugs into the cerebrospinal fluid. J. Pharmacol. Exp. Ther. 1960;1:20–25. - PubMed
-
- Yang H., Peters J.L., Michael A.C. Coupled effects of mass transfer and uptake kinetics on in vivo microdialysis of dopamine. J. Neurochem. 1998;2:684–692. - PubMed
-
- Syvanen S., Lindhe O., Palner M., Kornum B.R., Rahman O., Langstrom B., Knudsen G.M., Hammarlund-Udenaes M. Species differences in blood–brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab. Dispos. 2009;3:635–643. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

