ZASP interacts with the mechanosensing protein Ankrd2 and p53 in the signalling network of striated muscle
- PMID: 24647531
- PMCID: PMC3960238
- DOI: 10.1371/journal.pone.0092259
ZASP interacts with the mechanosensing protein Ankrd2 and p53 in the signalling network of striated muscle
Retraction in
-
Retraction: ZASP Interacts with the Mechanosensing Protein Ankrd2 and p53 in the Signalling Network of Striated Muscle.PLoS One. 2024 Jan 31;19(1):e0298249. doi: 10.1371/journal.pone.0298249. eCollection 2024. PLoS One. 2024. PMID: 38295040 Free PMC article. No abstract available.
Abstract
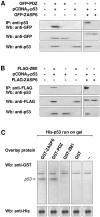
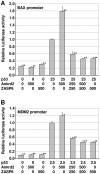
ZASP is a cytoskeletal PDZ-LIM protein predominantly expressed in striated muscle. It forms multiprotein complexes and plays a pivotal role in the structural integrity of sarcomeres. Mutations in the ZASP protein are associated with myofibrillar myopathy, left ventricular non-compaction and dilated cardiomyopathy. The ablation of its murine homologue Cypher results in neonatal lethality. ZASP has several alternatively spliced isoforms, in this paper we clarify the nomenclature of its human isoforms as well as their dynamics and expression pattern in striated muscle. Interaction is demonstrated between ZASP and two new binding partners both of which have roles in signalling, regulation of gene expression and muscle differentiation; the mechanosensing protein Ankrd2 and the tumour suppressor protein p53. These proteins and ZASP form a triple complex that appears to facilitate poly-SUMOylation of p53. We also show the importance of two of its functional domains, the ZM-motif and the PDZ domain. The PDZ domain can bind directly to both Ankrd2 and p53 indicating that there is no competition between it and p53 for the same binding site on Ankrd2. However there is competition for this binding site between p53 and a region of the ZASP protein lacking the PDZ domain, but containing the ZM-motif. ZASP is negative regulator of p53 in transactivation experiments with the p53-responsive promoters, MDM2 and BAX. Mutations in the ZASP ZM-motif induce modification in protein turnover. In fact, two mutants, A165V and A171T, were not able to bind Ankrd2 and bound only poorly to alpha-actinin2. This is important since the A165V mutation is responsible for zaspopathy, a well characterized autosomal dominant distal myopathy. Although the mechanism by which this mutant causes disease is still unknown, this is the first indication of how a ZASP disease associated mutant protein differs from that of the wild type ZASP protein.
Conflict of interest statement
Figures

Similar articles
-
Expression and Purification of ZASP Subdomains and Clinically Important Isoforms: High-Affinity Binding to G-Actin.Biochemistry. 2017 Apr 11;56(14):2061-2070. doi: 10.1021/acs.biochem.7b00067. Epub 2017 Apr 3. Biochemistry. 2017. PMID: 28349680 Free PMC article.
-
Z-disc-associated, alternatively spliced, PDZ motif-containing protein (ZASP) mutations in the actin-binding domain cause disruption of skeletal muscle actin filaments in myofibrillar myopathy.J Biol Chem. 2014 May 9;289(19):13615-26. doi: 10.1074/jbc.M114.550418. Epub 2014 Mar 25. J Biol Chem. 2014. PMID: 24668811 Free PMC article.
-
Zasp/Cypher internal ZM-motif containing fragments are sufficient to co-localize with alpha-actinin--analysis of patient mutations.Exp Cell Res. 2006 May 1;312(8):1299-311. doi: 10.1016/j.yexcr.2005.12.036. Epub 2006 Feb 14. Exp Cell Res. 2006. PMID: 16476425
-
"Z"eroing in on the role of Cypher in striated muscle function, signaling, and human disease.Trends Cardiovasc Med. 2007 Nov;17(8):258-62. doi: 10.1016/j.tcm.2007.09.002. Trends Cardiovasc Med. 2007. PMID: 18021935 Free PMC article. Review.
-
Ankrd2 in Mechanotransduction and Oxidative Stress Response in Skeletal Muscle: New Cues for the Pathogenesis of Muscular Laminopathies.Oxid Med Cell Longev. 2019 Jul 24;2019:7318796. doi: 10.1155/2019/7318796. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31428229 Free PMC article. Review.
Cited by
-
PDZ and LIM Domain-Encoding Genes: Their Role in Cancer Development.Cancers (Basel). 2023 Oct 19;15(20):5042. doi: 10.3390/cancers15205042. Cancers (Basel). 2023. PMID: 37894409 Free PMC article. Review.
-
Inherited cardiomyopathies.Circ J. 2014;78(10):2347-56. doi: 10.1253/circj.cj-14-0893. Epub 2014 Sep 2. Circ J. 2014. PMID: 25186923 Free PMC article. Review.
-
Downregulation of Cypher induces apoptosis in cardiomyocytes via Akt/p38 MAPK signaling pathway.Int J Med Sci. 2020 Aug 27;17(15):2328-2337. doi: 10.7150/ijms.48872. eCollection 2020. Int J Med Sci. 2020. PMID: 32922198 Free PMC article.
-
Differential expression and localization of Ankrd2 isoforms in human skeletal and cardiac muscles.Histochem Cell Biol. 2016 Nov;146(5):569-584. doi: 10.1007/s00418-016-1465-0. Epub 2016 Jul 8. Histochem Cell Biol. 2016. PMID: 27393496
-
Expression and Purification of ZASP Subdomains and Clinically Important Isoforms: High-Affinity Binding to G-Actin.Biochemistry. 2017 Apr 11;56(14):2061-2070. doi: 10.1021/acs.biochem.7b00067. Epub 2017 Apr 3. Biochemistry. 2017. PMID: 28349680 Free PMC article.
References
-
- Pyle WG, Solaro RJ (2004) At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res 94: 296–305. Review. - PubMed
-
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC (2002) Striated muscle cytoarchitecture: An intricate web of form and function. Annu Rev Cell Dev Biol 18: 637–706. - PubMed
-
- Sanger JM, Sanger JW (2008) The dynamic Z-band of striated muscle cells. Sci Signal 1: pe37. - PubMed
-
- Knöll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, et al. (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111: 943–955. - PubMed
-
- Frank D, Kuhn C, Katus HA, Frey N (2006) The sarcomeric Z-disc: a nodal point in signaling disease. J Mol Med 84: 446–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

