Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain
- PMID: 24647570
- PMCID: PMC4135174
- DOI: 10.1158/1078-0432.CCR-13-1941
Anti-VEGF antibodies mitigate the development of radiation necrosis in mouse brain
Abstract
Purpose: To quantify the effectiveness of anti-VEGF antibodies (bevacizumab and B20-4.1.1) as mitigators of radiation-induced, central nervous system (brain) necrosis in a mouse model.
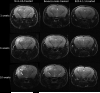
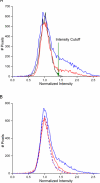
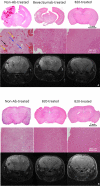
Experimental design: Cohorts of mice were irradiated with single-fraction 50- or 60-Gy doses of radiation targeted to the left hemisphere (brain) using the Leksell Perfexion Gamma Knife. The onset and progression of radiation necrosis were monitored longitudinally by in vivo, small-animal MRI, beginning 4 weeks after irradiation. MRI-derived necrotic volumes for antibody (Ab)-treated and untreated mice were compared. MRI results were supported by correlative histology.
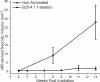
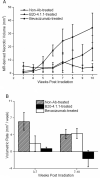
Results: Hematoxylin and eosin-stained sections of brains from irradiated, non-Ab-treated mice confirmed profound tissue damage, including regions of fibrinoid vascular necrosis, vascular telangiectasia, hemorrhage, loss of neurons, and edema. Treatment with the murine anti-VEGF antibody B20-4.1.1 mitigated radiation-induced changes in an extraordinary, highly statistically significant manner. The development of radiation necrosis in mice under treatment with bevacizumab (a humanized anti-VEGF antibody) was intermediate between that for B20-4.1.1-treated and non-Ab-treated animals. MRI findings were validated by histologic assessment, which confirmed that anti-VEGF antibody treatment dramatically reduced late-onset necrosis in irradiated brain.
Conclusions: The single-hemispheric irradiation mouse model, with longitudinal MRI monitoring, provides a powerful platform for studying the onset and progression of radiation necrosis and for developing and testing new therapies. The observation that anti-VEGF antibodies are effective mitigants of necrosis in our mouse model will enable a wide variety of studies aimed at dose optimization and timing and mechanism of action with direct relevance to ongoing clinical trials of bevacizumab as a treatment for radiation necrosis.
©2014 American Association for Cancer Research.
Figures
References
-
- Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9:180–8. - PubMed
-
- Stupp R, Mason WP, van den Beuf MJ. Radiotherapy plus concomitant and adjuvant temozolomide for newly diagnosed glioblastoma (vol 352, pg 19, 2005). Annals of Oncology. 2005;16:949.
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncology. 2009;10:459–66. - PubMed
-
- Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma-a review of the literature and current understanding. Acta Neurochirurgica. 2012;154:191–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

