Effect of silibinin in reducing inflammatory pathways in in vitro and in vivo models of infection-induced preterm birth
- PMID: 24647589
- PMCID: PMC3960267
- DOI: 10.1371/journal.pone.0092505
Effect of silibinin in reducing inflammatory pathways in in vitro and in vivo models of infection-induced preterm birth
Abstract
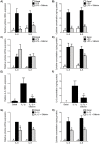
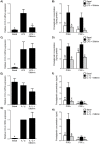
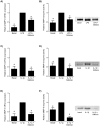
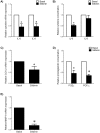
Infection-induced preterm birth is the largest cause of infant death and of neurological disabilities in survivors. Silibinin, from milk thistle, exerts potent anti-inflammatory activities in non-gestational tissues. The aims of this study were to determine the effect of silibinin on pro-inflammatory mediators in (i) human fetal membranes and myometrium treated with bacterial endotoxin lipopolysaccharide (LPS) or the pro-inflammatory cytokine IL-1β, and (ii) in preterm fetal membranes with active infection. The effect of silibinin on infection induced inflammation and brain injury in pregnant mice was also assessed. Fetal membranes and myometrium (tissue explants and primary cells) were treated with 200 μM silibinin in the presence or absence of 10 μg/ml LPS or 1 ng/ml IL-1β. C57BL/6 mice were injected with 70 mg/kg silibinin with or without 50 μg LPS on embryonic day 16. Fetal brains were collected after 6 h. In human fetal membranes, silibinin significantly decreased LPS-stimulated expression of IL-6 and IL-8, COX-2, and prostaglandins PGE2 and PGF2α. In primary amnion and myometrial cells, silibinin also decreased IL-1β-induced MMP-9 expression. Preterm fetal membranes with active infection treated with silibinin showed a decrease in IL-6, IL-8 and MMP-9 expression. Fetal brains from mice treated with silibinin showed a significant decrease in LPS-induced IL-8 and ninjurin, a marker of brain injury. Our study demonstrates that silibinin can reduce infection and inflammation-induced pro-labour mediators in human fetal membranes and myometrium. Excitingly, the in vivo results indicate a protective effect of silibinin on infection-induced brain injury in a mouse model of preterm birth.
Conflict of interest statement
Figures
References
-
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, et al. (2001) Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 185: 1130–1136. - PubMed
-
- Menon R, Fortunato SJ (2007) Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol 21: 467–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

