Pro-inflammatory mediation of myoblast proliferation
- PMID: 24647690
- PMCID: PMC3960233
- DOI: 10.1371/journal.pone.0092363
Pro-inflammatory mediation of myoblast proliferation
Abstract
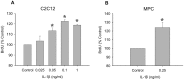
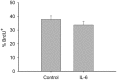
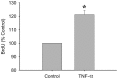
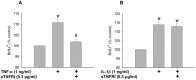
Skeletal muscle satellite cell function is largely dictated by the surrounding environment following injury. Immune cell infiltration dominates the extracellular space in the injured area, resulting in increased cytokine concentrations. While increased pro-inflammatory cytokine expression has been previously established in the first 3 days following injury, less is known about the time course of cytokine expression and the specific mechanisms of cytokine induced myoblast function. Therefore, the expression of IL-1β and IL-6 at several time points following injury, and their effects on myoblast proliferation, were examined. In order to do this, skeletal muscle was injured using barium chloride in mice and tissue was collected 1, 5, 10, and 28 days following injury. Mechanisms of cytokine induced proliferation were determined in cell culture using both primary and C2C12 myoblasts. It was found that there is a ∼20-fold increase in IL-1β (p≤0.05) and IL-6 (p = 0.06) expression 5 days following injury. IL-1β increased proliferation of both primary and C2C12 cells ∼25%. IL-1β stimulation also resulted in increased NF-κB activity, likely contributing to the increased proliferation. These data demonstrate for the first time that IL-1β alone can increase the mitogenic activity of primary skeletal muscle satellite cells and offer insight into the mechanisms dictating satellite cell function following injury.
Conflict of interest statement
Figures




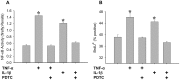
References
-
- Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551. - PubMed
-
- Parise G, McKinnell IW, Rudnicki MA (2008) Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve 37: 611–619. - PubMed
-
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, et al. (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

