Cells of renin lineage take on a podocyte phenotype in aging nephropathy
- PMID: 24647714
- PMCID: PMC4024732
- DOI: 10.1152/ajprenal.00699.2013
Cells of renin lineage take on a podocyte phenotype in aging nephropathy
Abstract
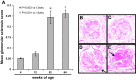
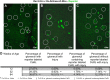
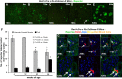
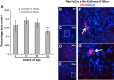
Aging nephropathy is characterized by podocyte depletion accompanied by progressive glomerulosclerosis. Replacement of terminally differentiated podocytes by local stem/progenitor cells is likely a critical mechanism for their regeneration. Recent studies have shown that cells of renin lineage (CoRL), normally restricted to the kidney's extraglomerular compartment, might serve this role after an abrupt depletion in podocyte number. To determine the effects of aging on the CoRL reserve and if CoRL moved from an extra- to the intraglomerular compartment during aging, genetic cell fate mapping was performed in aging Ren1cCre × Rs-ZsGreen reporter mice. Podocyte number decreased and glomerular scarring increased with advanced age. CoRL number decreased in the juxtaglomerular compartment with age. There was a paradoxical increase in CoRL in the intraglomerular compartment at 52 and 64 wk of age, where a subset coexpressed the podocyte proteins nephrin, podocin, and synaptopodin. Transmission electron microscopy studies showed that a subset of labeled CoRL in the glomerulus displayed foot processes, which attached to the glomerular basement membrane. No CoRL in the glomerular compartment stained for renin. These results suggest that, despite a decrease in the reserve, a subpopulation of CoRL moves to the glomerulus after chronic podocyte depletion in aging nephropathy, where they acquire a podocyte-like phenotype. This suggests that they might serve as adult podocyte stem/progenitor cells under these conditions, albeit in insufficient numbers to fully replace podocytes depleted with age.
Keywords: cells of renin lineage; focal segmental glomerulosclerosis; glomerulus; podocyte; regeneration.
Copyright © 2014 the American Physiological Society.
Figures



Comment in
-
Re: Cells of renin lineage take on a podocyte phenotype in aging nephropathy.J Urol. 2015 Feb;193(2):731. doi: 10.1016/j.juro.2014.11.022. Epub 2014 Nov 13. J Urol. 2015. PMID: 25617310 No abstract available.
-
Bridges to cross, burn, and mend: cells of renin lineage as podocyte progenitors.Am J Physiol Renal Physiol. 2015 Sep 15;309(6):F499-500. doi: 10.1152/ajprenal.00301.2015. Epub 2015 Jul 15. Am J Physiol Renal Physiol. 2015. PMID: 26180240 No abstract available.
References
-
- Alpers CE, Hudkins KL, Floege J, Johnson RJ. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol 5: 201–209, 1994 - PubMed
-
- Angelotti ML, Lazzeri E, Lasagni L, Romagnani P. Only anti-CD133 antibodies recognizing the CD133/1 or the CD133/2 epitopes can identify human renal progenitors. Kidney Int 78: 620–621, 2010 - PubMed
-
- Brinkkoetter PT, Wu JS, Ohse T, Krofft RD, Schermer B, Benzing T, Pippin JW, Shankland SJ. p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int 77: 690–699, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

