Erythropoietin signaling: a novel regulator of white adipose tissue inflammation during diet-induced obesity
- PMID: 24647735
- PMCID: PMC4066343
- DOI: 10.2337/db13-0883
Erythropoietin signaling: a novel regulator of white adipose tissue inflammation during diet-induced obesity
Abstract
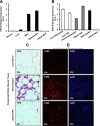
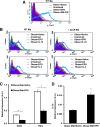
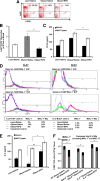
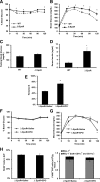
Obesity-induced white adipose tissue (WAT) inflammation and insulin resistance are associated with macrophage (Mф) infiltration and phenotypic shift from "anti-inflammatory" M2-like to predominantly "proinflammatory" M1-like cells. Erythropoietin (EPO), a glycoprotein hormone indispensable for erythropoiesis, has biological activities that extend to nonerythroid tissues, including antiapoptotic and anti-inflammatory effects. Using comprehensive in vivo and in vitro analyses in mice, EPO treatment inhibited WAT inflammation, normalized insulin sensitivity, and reduced glucose intolerance. We investigated EPO receptor (EPO-R) expression in WAT and characterized the role of its signaling during obesity-induced inflammation. Remarkably, and prior to any detectable changes in body weight or composition, EPO treatment reduced M1-like Mф and increased M2-like Mф in WAT, while decreasing inflammatory monocytes. These anti-inflammatory effects were found to be driven, at least in part, by direct EPO-R response in Mф via Stat3 activation, where EPO effects on M2 but not M1 Mф required interleukin-4 receptor/Stat6. Using obese ∆EpoR mice with EPO-R restricted to erythroid cells, we demonstrated an anti-inflammatory role for endogenous EPO. Collectively, our findings identify EPO-R signaling as a novel regulator of WAT inflammation, extending its nonerythroid activity to encompass effects on both Mф infiltration and subset composition in WAT.
© 2014 by the American Diabetes Association.
Figures








Comment in
-
Beyond erythropoiesis: emerging metabolic roles of erythropoietin.Diabetes. 2014 Jul;63(7):2229-31. doi: 10.2337/db14-0566. Diabetes. 2014. PMID: 24962925 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

