Localization of trehalose in partially hydrated DOPC bilayers: insights into cryoprotective mechanisms
- PMID: 24647907
- PMCID: PMC4006246
- DOI: 10.1098/rsif.2014.0069
Localization of trehalose in partially hydrated DOPC bilayers: insights into cryoprotective mechanisms
Abstract
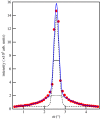
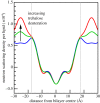
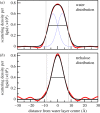
Trehalose, a natural disaccharide with bioprotective properties, is widely recognized for its ability to preserve biological membranes during freezing and dehydration events. Despite debate over the molecular mechanisms by which this is achieved, and that different mechanisms imply quite different distributions of trehalose molecules with respect to the bilayer, there are no direct experimental data describing the location of trehalose within lipid bilayer membrane systems during dehydration. Here, we use neutron membrane diffraction to conclusively show that the trehalose distribution in a dioleoylphosphatidylcholine (DOPC) system follows a Gaussian profile centred in the water layer between bilayers. The absence of any preference for localizing near the lipid headgroups of the bilayers indicates that the bioprotective effects of trehalose at physiologically relevant concentrations are the result of non-specific mechanisms that do not rely on direct interactions with the lipid headgroups.
Keywords: anhydrobiology; cryobiology; cryoprotection; lipid membrane; membrane diffraction.
Figures

References
-
- Koster KL, Webb MS, Bryant G, Lynch DV. 1994. Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: vitrification of sugars alters the phase behavior of the phospholipid. Biochim. Biophys. Acta Biomembr. 1193, 143–150. ( 10.1016/0005-2736(94)90343-3) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
