Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting"
- PMID: 24647944
- PMCID: PMC3960467
- DOI: 10.1523/JNEUROSCI.4458-13.2014
Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting"
Abstract
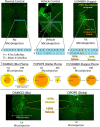
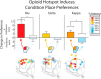
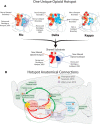
A specialized cubic-millimeter hotspot in the rostrodorsal quadrant of medial shell in nucleus accumbens (NAc) of rats may mediate opioid enhancement of gustatory hedonic impact or "liking". Here, we selectively stimulated the three major subtypes of opioid receptors via agonist microinjections [mu (DAMGO), delta (DPDPE), or kappa (U50488H)] and constructed anatomical maps for functional localizations of consequent changes in hedonic "liking" (assessed by affective orofacial reactions to sucrose taste) versus "wanting" (assessed by changes in food intake). Results indicated that the NAc rostrodorsal quadrant contains a shared opioid hedonic hotspot that similarly mediates enhancements of sucrose "liking" for mu, delta, and kappa stimulations. Within the rostrodorsal hotspot boundaries each type of stimulation generated at least a doubling or higher enhancement of hedonic reactions, with comparable intensities for all three types of opioid stimulation. By contrast, a negative hedonic coldspot was mapped in the caudal half of medial shell, where all three types of opioid stimulation suppressed "liking" reactions to approximately one-half normal levels. Different anatomical patterns were produced for stimulation of food "wanting", reflected in food intake. Altogether, these results indicate that the rostrodorsal hotspot in medial shell is unique for generating opioid-induced hedonic enhancement, and add delta and kappa signals to mu as hedonic generators within the hotspot. Also, the identification of a separable NAc caudal coldspot for hedonic suppression, and separate NAc opioid mechanisms for controlling food "liking" versus "wanting" further highlights NAc anatomical heterogeneity and localizations of function within subregions of medial shell.
Keywords: nucleus accumbens; opioid.
Figures

References
-
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. - PubMed
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
