Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles
- PMID: 24647946
- PMCID: PMC6608097
- DOI: 10.1523/JNEUROSCI.3192-13.2014
Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles
Abstract
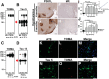
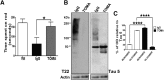
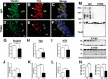
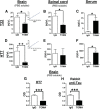
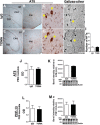
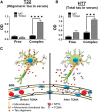
Recent findings suggest that tau oligomers, which form before neurofibrillary tangles (NFTs), are the true neurotoxic tau entities in neurodegenerative tauopathies, including Alzheimer's disease (AD). Studies in animal models of tauopathy suggest that tau oligomers play a key role in eliciting behavioral and cognitive impairments. Here, we used a novel tau oligomer-specific monoclonal antibody (TOMA) for passive immunization in mice expressing mutant human tau. A single dose of TOMA administered either intravenously or intracerebroventricularly was sufficient to reverse both locomotor and memory deficits in a mouse model of tauopathy for 60 d, coincident with rapid reduction of tau oligomers but not phosphorylated NFTs or monomeric tau. Our data demonstrate that antibody protection is mediated by extracellular and rapid peripheral clearance. These findings provide the first direct evidence in support of a critical role for tau oligomers in disease progression and validate tau oligomers as a target for the treatment of AD and other neurodegenerative tauopathies.
Keywords: Alzheimer's disease; immunotherapy; tau oligomers; tauopathies.
Figures



References
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
