Parametric alpha- and beta-band signatures of supramodal numerosity information in human working memory
- PMID: 24647949
- PMCID: PMC6608098
- DOI: 10.1523/JNEUROSCI.4580-13.2014
Parametric alpha- and beta-band signatures of supramodal numerosity information in human working memory
Abstract
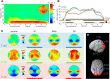
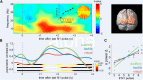
Numerosity can be assessed by analog estimation, similar to a continuous magnitude, or by discrete quantification of the individual items in a set. While the extent to which these two processes rely on common neural mechanisms remains debated, recent studies of sensory working memory (WM) have identified an oscillatory signature of continuous magnitude information, in terms of quantitative modulations of prefrontal upper beta activity (20-30 Hz). Here, we examined how such parametric oscillatory WM activity may also reflect the abstract assessment of the numerosity of discrete items. We recorded EEG while participants (n = 24) processed the number of stimulus pulses presented in the visual, auditory, or tactile modality, under otherwise identical experimental conditions. Behavioral response profiles showed varying degrees of analog estimation and of discretized quantification in the different modalities. During sustained processing in WM, the amplitude of posterior alpha oscillations (8-13 Hz) reflected the increased memory load associated with maintaining larger sets of discrete items. In contrast, earlier numerosity-dependent modulations of right prefrontal upper beta (20-30 Hz) specifically reflected the extent to which numerosity was assessed by analog estimation, both between and within presentation modalities. Together, the analog approximation-but not the discretized representation-of numerosity information exhibited a parametric oscillatory signature akin to a continuous sensory magnitude. The results suggest dissociable oscillatory mechanisms of abstract numerosity integration, at a supramodal processing stage in human WM.
Keywords: EEG; neural coding; numerical cognition; oscillations; prefrontal cortex.
Figures




References
-
- Dehaene S. The number sense how the mind creates mathematics. New York: Oxford UP; 1997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
