EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation
- PMID: 24648348
- PMCID: PMC4278592
- DOI: 10.1158/0008-5472.CAN-13-3157
EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation
Abstract
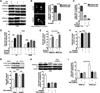
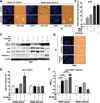
Therapeutics that target the epidermal growth factor receptor (EGFR) can enhance the cytotoxic effects of ionizing radiation (IR). However, predictive genomic biomarkers of this radiosensitization have remained elusive. By screening 40 non-small cell lung cancer cell (NSCLC) lines, we established a surprising positive correlation between the presence of a KRAS mutation and radiosensitization by the EGFR inhibitors erlotinib and cetuximab. EGFR signaling in KRAS-mutant NSCLC cells promotes chromatin condensation in vitro and in vivo, thereby restricting the number of DNA double-strand breaks (DSB) produced by a given dose of IR. Chromatin condensation in interphase cells is characterized by an unexpected mitosis-like colocalization of serine 10 phosphorylation and lysine 9 trimethylation on histone H3. Aurora B promotes this process in a manner that is codependent upon EGFR and protein kinase C α (PKCα). PKCα, in addition to MEK/ERK signaling, is required for the suppression of DSB-inducible premature senescence by EGFR. Blockade of autophagy results in a mutant KRAS-dependent senescence-to-apoptosis switch in cancer cells treated with IR and erlotinib. In conclusion, we identify EGFR as a molecular target to overcome a novel mechanism of radioresistance in KRAS-mutant tumor cells, which stands in contrast to the unresponsiveness of KRAS-mutant cancers to EGFR-directed agents in monotherapy. Our findings may reposition EGFR-targeted agents for combination with DSB-inducing therapies in KRAS-mutant NSCLC.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. - PubMed
-
- Kegel P, Riballo E, Kuhne M, Jeggo PA, Lobrich M. X-irradiation of cells on glass slides has a dose doubling impact. DNA Repair (Amst) 2007;6:1692–1697. - PubMed
-
- Willers H, Held KD. Introduction to clinical radiation biology. Hematol Oncol Clin North Am. 2006;20:1–24. - PubMed
-
- Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE, Chakraborty C, Barreto-Andrade JC, Crawley C, Sutton HG, Kron SJ, Weichselbaum RR. Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res. 2010;70:6277–6282. - PMC - PubMed
-
- Toulany M, Kasten-Pisula U, Brammer I, Wang S, Chen J, Dittmann K, Baumann M, Dikomey E, Rodemann HP. Blockage of epidermal growth factor receptor-phosphatidylinositol 3-kinase-AKT signaling increases radiosensitivity of K-RAS mutated human tumor cells in vitro by affecting DNA repair. Clin Cancer Res. 2006;12:4119–4126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

