Comparative study analyzing survival and safety of bevacizumab/carboplatin/paclitaxel versus carboplatin/docetaxel in initial treatment of metastatic Her-2-negative breast cancer
- PMID: 24648756
- PMCID: PMC3929329
- DOI: 10.2147/BCTT.S44728
Comparative study analyzing survival and safety of bevacizumab/carboplatin/paclitaxel versus carboplatin/docetaxel in initial treatment of metastatic Her-2-negative breast cancer
Abstract
Purpose: In view of the previous reports demonstrating the positive outcome of bevacizumab in metastatic breast cancer, we aimed at comparing the role of bevacizumab-based metronomic combination with taxane (paclitaxel) versus a different taxane (docetaxel)-based regimen in addition to carboplatin as initial treatment for metastatic Her-2-negative breast cancer.
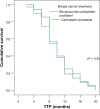
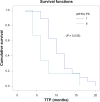
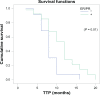
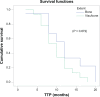
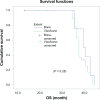
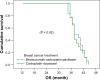
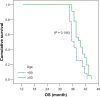
Patients and methods: This is a randomized Phase III study comparing the progression-free survival (PFS) and safety in Her-2-negative female patients with initial diagnosis of metastatic breast cancer with World Health Organization performance status of 0-II. Forty-one patients were randomized from September 2008 to July 2009 to receive either; (1) bevacizumab 5 mg/kg day 1 and day 15, carboplatin area under the curve (AUC)-2 day 1, day 8, and day 15, and paclitaxel 60 mg/m(2) day 1, day 8, and day 15 (arm-I); or (2) carboplatin AUC-5 day 1, docetaxel 75 mg/m(2) day 1 (arm-II). The Kaplan-Meier method was used for estimating survival; log-rank test for comparing survival curves. The primary end point was PFS, and secondary end points were overall survival (OS) and safety.
Results: PFS was 10 months in arm I versus 10.2 months in arm II (P = 0.9). The OS rate was similar in both arms: 37.6 months for arm I versus 37.4 months for arm II (P = 0.92). The toxicity revealed higher incidence of hypertension and proteinuria in arm I; however, with higher incidence of grade III-IV neutropenia and neutropenic fever in arm II. No treatment-related mortality was recorded.
Conclusion: Bevacizumab/carboplatin/paclitaxel and carboplatin/docetaxel show comparable PFS and OS with different toxicity profiles.
Keywords: Her-2 negative; bevacizumab; breast cancer; taxanes.
Figures
References
-
- American Cancer Society . Cancer Facts and Figures 2003. Atlanta, GA: American Cancer Society; 2003.
-
- Mincey BA, Perez EA. Concise review for clinicians: advances in screening, diagnosis and treatment of breast cancer. Mayo Clin Proc. 2004;79:810–816. - PubMed
-
- Carlson RW, Anderson BO, Bensinger W, et al. NCCN Practice Guidelines in Oncology, Version 2. National Comprehensive Cancer Network; 2002.
-
- Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. - PubMed
-
- Fountzilas G, Athanassiades A, Papadimitriou V, et al. Paclitaxel and carboplatin as first-line chemotherapy for advanced breast cancer. Oncology (Huntingt) 1998;12(Suppl 1):45–48. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

