Marine- and plant-derived ω-3 fatty acids differentially regulate prostate cancer cell proliferation
- PMID: 24649190
- PMCID: PMC3916163
- DOI: 10.3892/mco.2013.76
Marine- and plant-derived ω-3 fatty acids differentially regulate prostate cancer cell proliferation
Abstract
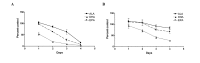
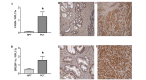
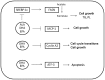
Fish oil contains the marine ω-3 polyunsaturated fatty acids (ω-3 PUFAs) docosahexaenoic (DHA) and eicosapentaenoic acid (EPA). The consumption of diets rich in these fatty acids is associated with a decreased incidence of prostate cancer. However, there is limited knowledge regarding the non-marine ω-3 PUFA α-linolenic acid (ALA). To study which ω-3 PUFAs are more effective in prostate cancer prevention, and whether the mechanisms of action are conserved between them, we investigated the effect of DHA, EPA and ALA on the human prostate cancer cell lines PC-3 and LNCaP. Different trends of inhibition of PC-3 cell proliferation were observed for the three ω-3 PUFA, with DHA having the most pronounced effects on cell proliferation, while ALA had the minimum effects of the three ω-3 PUFAs. All the ω-3 PUFAs decreased fatty acid synthase (FASN) mRNA. Concerning genes involved in inflammation, cell cycle and apoptosis, DHA regulated the most genes in all categories, followed by EPA and then ALA. In addition, DHA and EPA increased the gene expression of the pro-apoptotic protein activating transcription factor 3 mRNA. Moreover, these two fatty acids significantly induced apoptosis. In conclusion, while some mechanisms of cancer cell inhibition are conserved among ω-3 PUFA, the extent, magnitude, and duration of transcriptional changes vary for each individual fatty acid.
Keywords: docosahexaenoic acid; eicosapentaenoic acid; prostate cancer; α-linolenic acid; ω-3.
Figures


References
-
- Rambeaud JJ. Intermittent complete androgen blockade in meta-static prostate cancer. Eur Urol. 1999;35(Suppl 1):32–36. - PubMed
-
- Harris KA, Reese DM. Treatment options in hormone-refractory prostate cancer: current and future approaches. Drugs. 2001;61:2177–2192. - PubMed
-
- Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23:8152–8160. - PubMed
-
- King IB, Kristal AR, Schaffer S, Thornquist M, Goodman GE. Serum trans-fatty acids are associated with risk of prostate cancer in beta-Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2005;14:988–992. - PubMed
-
- Haaland CM, Heaphy CM, Butler KS, Fischer EG, Griffith JK, Bisoffi M. Differential gene expression in tumor adjacent histologically normal prostatic tissue indicates field cancerization. Int J Oncol. 2009;35:537–546. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
