What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health
- PMID: 24650218
- PMCID: PMC4049420
- DOI: 10.1111/nyas.12327
What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health
Abstract
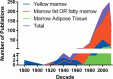
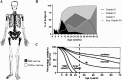
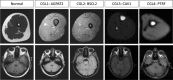
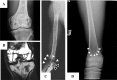
Marrow adipose tissue (MAT) is functionally distinct from both white and brown adipose tissue and can contribute to systemic and skeletal metabolism. MAT formation is a spatially and temporally defined developmental event, suggesting that MAT is an organ that serves important functions and, like other organs, can undergo pathologic change. The well-documented inverse relationship between MAT and bone mineral density has been interpreted to mean that MAT removal is a possible therapeutic target for osteoporosis. However, the bone and metabolic phenotypes of patients with lipodystrophy argues that retention of MAT may actually be beneficial in some circumstances. Furthermore, MAT may exist in two forms, regulated and constitutive, with divergent responses to hematopoietic and nutritional demands. In this review, we discuss the role of MAT in lipodystrophy, bone loss, and metabolism, and highlight our current understanding of this unique adipose tissue depot.
Keywords: diabetes; lipodystrophy; marrow adipose tissue; marrow fat; osteoporosis.
© 2014 New York Academy of Sciences.
Figures


References
-
- Ranvier LA. Traite d'histologie. Paris, F. Savy. 319:1875–1878.
-
- Piney A. The anatomy of the bone marrow. Br. Med. J. 1922;2:792–795.
-
- Custer RP. Studies on the structure and function of bone marrow part I. J. Lab. Clin. Med. 1932;17:951–960.
-
- Custer RP, Ahlfeldt FE. Studies on the Structure and Function of Bone Marrow II. J. Lab. Clin. Med. 1932;17:960–962.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

