Preconception maternal nutrition: a multi-site randomized controlled trial
- PMID: 24650219
- PMCID: PMC4000057
- DOI: 10.1186/1471-2393-14-111
Preconception maternal nutrition: a multi-site randomized controlled trial
Abstract
Background: Research directed to optimizing maternal nutrition commencing prior to conception remains very limited, despite suggestive evidence of its importance in addition to ensuring an optimal nutrition environment in the periconceptional period and throughout the first trimester of pregnancy.
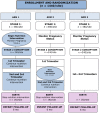
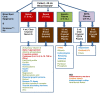
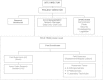
Methods/study design: This is an individually randomized controlled trial of the impact on birth length (primary outcome) of the time at which a maternal nutrition intervention is commenced: Arm 1: ≥ 3 mo preconception vs. Arm 2: 12-14 wk gestation vs. Arm 3: none.192 (derived from 480) randomized mothers and living offspring in each arm in each of four research sites (Guatemala, India, Pakistan, Democratic Republic of the Congo). The intervention is a daily 20 g lipid-based (118 kcal) multi-micronutient (MMN) supplement. Women randomized to receive this intervention with body mass index (BMI) <20 or whose gestational weight gain is low will receive an additional 300 kcal/d as a balanced energy-protein supplement. Researchers will visit homes biweekly to deliver intervention and monitor compliance, pregnancy status and morbidity; ensure prenatal and delivery care; and promote breast feeding. The primary outcome is birth length. Secondary outcomes include: fetal length at 12 and 34 wk; incidence of low birth weight (LBW); neonatal/infant anthropometry 0-6 mo of age; infectious disease morbidity; maternal, fetal, newborn, and infant epigenetics; maternal and infant nutritional status; maternal and infant microbiome; gut inflammatory biomarkers and bioactive and nutritive compounds in breast milk. The primary analysis will compare birth Length-for-Age Z-score (LAZ) among trial arms (independently for each site, estimated effect size: 0.35). Additional statistical analyses will examine the secondary outcomes and a pooled analysis of data from all sites.
Discussion: Positive results of this trial will support a paradigm shift in attention to nutrition of all females of child-bearing age.
Trial registration: ClinicalTrials.gov NCT01883193.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical