Simultaneous determination of all forms of biopterin and neopterin in cerebrospinal fluid
- PMID: 24650440
- PMCID: PMC4102970
- DOI: 10.1021/cn4001928
Simultaneous determination of all forms of biopterin and neopterin in cerebrospinal fluid
Abstract
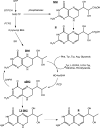
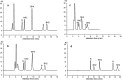
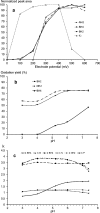
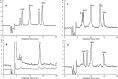
In humans, genetic defects of the synthesis or regeneration of tetrahydrobiopterin (BH4), an essential cofactor in hydroxylation reactions, are associated with severe neurological disorders. The diagnosis of these conditions relies on the determination of BH4, dihydrobiopterin (BH2), and dihydroneopterin (NH2) in cerebrospinal fluid (CSF). As MS/MS is less sensitive than fluorescence detection (FD) for this purpose, the most widely used method since 1980 involves two HPLC runs including two differential off-line chemical oxidation procedures aiming to transform the reduced pterins into their fully oxidized fluorescent counterparts, biopterin (B) and neopterin (N). However, this tedious and time-consuming two-step indirect method underestimates BH4, BH2, and NH2 concentrations. Direct quantification of BH4 is essential for studying its metabolism and for monitoring the efficacy of BH4 supplementation in patients with genetic defects. Here we describe a single step method to simultaneously measure BH4, BH2, B, NH2, and N in CSF by HPLC coupled to FD after postcolumn coulometric oxidation. All target pterins were quantified in CSF with a small volume (100 μL), and a single filtration step for sample preparation and analysis. As compared to the most widely used method in more than 100 CSF samples, this new assay is the easiest route for accurately determining in a single run BH4, BH2, and NH2 in CSF in deficit situations as well as for monitoring the efficacy of the treatment.
Figures




Similar articles
-
Cerebrospinal Fluid Pterins, Pterin-Dependent Neurotransmitters, and Mortality in Pediatric Cerebral Malaria.J Infect Dis. 2021 Oct 28;224(8):1432-1441. doi: 10.1093/infdis/jiab086. J Infect Dis. 2021. PMID: 33617646 Free PMC article.
-
High-performance liquid chromatographic measurement of cerebrospinal fluid tetrahydrobiopterin, neopterin, homovanillic acid and 5-hydroxindoleacetic acid in neurological diseases.J Chromatogr B Biomed Appl. 1994 Jul 1;657(1):61-6. doi: 10.1016/0378-4347(94)80070-7. J Chromatogr B Biomed Appl. 1994. PMID: 7524948
-
Determination of marker pteridins and biopterin reduced forms, tetrahydrobiopterin and dihydrobiopterin, in human urine, using a post-column photoinduced fluorescence liquid chromatographic derivatization method.Anal Chim Acta. 2009 Aug 19;648(1):113-22. doi: 10.1016/j.aca.2009.06.045. Epub 2009 Jun 24. Anal Chim Acta. 2009. PMID: 19616696
-
Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4.Mol Genet Metab. 2005 Dec;86 Suppl 1:S2-10. doi: 10.1016/j.ymgme.2005.09.002. Epub 2005 Oct 25. Mol Genet Metab. 2005. PMID: 16256391 Review.
-
[Biopterin and child neurologic disease].No To Hattatsu. 2009 Jan;41(1):5-10. No To Hattatsu. 2009. PMID: 19172809 Review. Japanese.
Cited by
-
Older-onset levodopa-responsive parkinsonism with normal DAT-SPECT and pterin hypometabolism.BMJ Case Rep. 2021 May 7;14(5):e240067. doi: 10.1136/bcr-2020-240067. BMJ Case Rep. 2021. PMID: 33962918 Free PMC article.
-
What Have We Learned from Cerebrospinal Fluid Studies about Biomarkers for Detecting LRRK2 Parkinson's Disease Patients and Healthy Subjects with Parkinson's-Associated LRRK2 Mutations?J Parkinsons Dis. 2019;9(3):467-488. doi: 10.3233/JPD-191630. J Parkinsons Dis. 2019. PMID: 31322581 Free PMC article. Review.
-
Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor.Antioxidants (Basel). 2023 May 3;12(5):1037. doi: 10.3390/antiox12051037. Antioxidants (Basel). 2023. PMID: 37237903 Free PMC article. Review.
-
Autoxidation Kinetics of Tetrahydrobiopterin-Giving Quinonoid Dihydrobiopterin the Consideration It Deserves.Molecules. 2023 Jan 28;28(3):1267. doi: 10.3390/molecules28031267. Molecules. 2023. PMID: 36770933 Free PMC article.
-
LC-MS/MS Analysis of Cerebrospinal Fluid Metabolites in the Pterin Biosynthetic Pathway.JIMD Rep. 2016;29:1-9. doi: 10.1007/8904_2014_336. Epub 2014 Sep 12. JIMD Rep. 2016. PMID: 25213568 Free PMC article.
References
-
- Koshimura K.; Murakami Y.; Tanaka J.; Kato Y. (2000) The role of 6R-tetrahydrobiopterin in the nervous system. Prog. Neurobiol. 61, 415–438. - PubMed
-
- Hoekstra R.; Fekkes D. (2002) Pteridines and affective disorders. Acta Neuropsychiatr. 14, 120–126. - PubMed
-
- Blau N., and Thöny B. (2008) Pterins and related enzymes. In Laboratory guide to the methods in biochemical genetics (Blau N., Duran M., and Gibson K. M., Eds.), pp 665–701, Springer, Berlin, Heidelberg.
-
- Fukushima T.; Nixon J. C. (1980) Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 102, 176–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

