Neural integration of satiation and food reward: role of GLP-1 and orexin pathways
- PMID: 24650552
- PMCID: PMC4167985
- DOI: 10.1016/j.physbeh.2014.03.013
Neural integration of satiation and food reward: role of GLP-1 and orexin pathways
Abstract
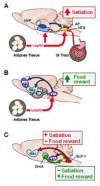
Central nervous system control of food intake involves detecting, integrating and responding to diverse internal and external signals. For maintenance of energy homeostasis, the brain uses long-term signals of metabolic status and short-term signals related to the nutrient content of individual meals. Feeding is also clearly influenced by hedonic, reward-related factors: palatability, motivation, and learned associations and cues that predict the availability of food. Different neural circuits have been proposed to mediate these homeostatic and hedonic aspects of eating. This review describes research on neural pathways that appear to be involved in both, integrating gastrointestinal satiation signaling with food reward. First, the glucagon-like peptide 1 projections from the nucleus of the solitary tract to the nucleus accumbens and ventral tegmental area are discussed as a mechanism through which meal-related gut signals may influence palatability, motivation for food, and meal size. Second, the orexin projection from lateral hypothalamus to the nucleus of the solitary tract and area postrema is discussed as a mechanism through which cues that predict rewarding food may act to increase motivation for food and also to suppress satiation. Additional potential integrative sites and pathways are also briefly discussed. Based on these findings, it is suggested that the brain circuitry involved in energy homeostasis and the circuitry mediating food reward are, in fact, overlapping and far less distinct than previously considered.
Keywords: Food reward; Glucagon-like peptide 1; Orexin; Satiation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

