Sitagliptin prevents aggravation of endocrine and exocrine pancreatic damage in the Zucker Diabetic Fatty rat - focus on amelioration of metabolic profile and tissue cytoprotective properties
- PMID: 24650557
- PMCID: PMC3998187
- DOI: 10.1186/1758-5996-6-42
Sitagliptin prevents aggravation of endocrine and exocrine pancreatic damage in the Zucker Diabetic Fatty rat - focus on amelioration of metabolic profile and tissue cytoprotective properties
Abstract
Background: The purpose of this study was to investigate some of the possible mechanisms underlying the protective effects of a dipeptidyl peptidase IV (DPP-IV) inhibitor, sitagliptin, on pancreatic tissue in an animal model of type 2 diabetes mellitus (T2DM), the Zucker Diabetic Fatty (ZDF) rat, focusing on glycaemic, insulinic and lipidic profiles, as well as, on apoptosis, inflammation, angiogenesis and proliferation mediators.
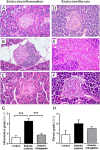
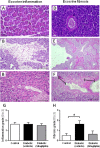
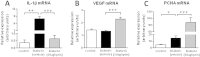
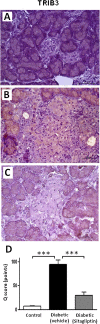
Methods: Male obese diabetic ZDF (fa/fa) rats, aged 20 weeks, were treated with sitagliptin (10 mg/kg bw/day) during 6 weeks and compared to untreated diabetic and lean control littermates. Metabolic data was evaluated at the beginning and at the end of the treatment, including glycaemia, HbA1c, insulinaemia, HOMA-beta and TGs. Endocrine and exocrine pancreas lesions were assessed semiquantitatively by histopathological methods. Pancreas gene (mRNA) and protein expression of mediators of apoptotic machinery, inflammation and angiogenesis/proliferation (Bax, Bcl2, IL-1β, VEGF, PCNA and TRIB3) were analyzed by RT-qPCR and/or by immunohistochemistry.
Results: Sitagliptin treatment for 6 weeks (between 20 and 26 week-old) was able to significantly (p < 0.001) ameliorate all the metabolic parameters, by preventing the increase in blood glucose and in serum TGs contents (16.54% and 37.63%, respectively, vs untreated), as well as, by preventing the decrease in serum insulin levels and in the functional beta cells capacity accessed via HOMA-beta index (156.28% and 191.74%, respectively, vs untreated). Sitagliptin-treated diabetic rats presented a reduced pancreas Bax/Bcl2 ratio, suggestive of an antiapoptotic effect; in addition, sitagliptin was able to completely reduce (p < 0.001) the pancreas overexpression of IL-1β and TRIB3 found in the untreated diabetic animals; and promoted a significant (p < 0.001) overexpression of VEGF and PCNA.
Conclusion: In this animal model of obese T2DM (the ZDF rat), sitagliptin prevented β-cell dysfunction and evolution of pancreatic damage. The protective effects afforded by this DPP-IV inhibitor may derive from improvement of the metabolic profile (viewed by the amelioration of glucose and TGs levels and of insulin resistance) and from cytoprotective properties, such as antiapoptotic, anti-inflammatory, pro-angiogenic and pro-proliferative.
Figures

Similar articles
-
Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals.Mediators Inflamm. 2014;2014:538737. doi: 10.1155/2014/538737. Epub 2014 Apr 8. Mediators Inflamm. 2014. PMID: 24817793 Free PMC article.
-
Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat).Mediators Inflamm. 2010;2010:592760. doi: 10.1155/2010/592760. Epub 2010 Jun 21. Mediators Inflamm. 2010. PMID: 20652060 Free PMC article.
-
Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat).Exp Diabetes Res. 2011;2011:162092. doi: 10.1155/2011/162092. Epub 2011 Nov 30. Exp Diabetes Res. 2011. PMID: 22203828 Free PMC article.
-
Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model.Diabetes Obes Metab. 2012 May;14(5):454-63. doi: 10.1111/j.1463-1326.2011.01548.x. Epub 2012 Jan 6. Diabetes Obes Metab. 2012. PMID: 22151893
-
Characterization of the exocrine pancreas in the male Zucker diabetic fatty rat model of type 2 diabetes mellitus following 3 months of treatment with sitagliptin.Endocrinology. 2014 Mar;155(3):783-92. doi: 10.1210/en.2013-1781. Epub 2014 Jan 1. Endocrinology. 2014. PMID: 24424056
Cited by
-
DPP-4 Inhibitors: Renoprotective Potential and Pharmacokinetics in Type 2 Diabetes Mellitus Patients with Renal Impairment.Eur J Drug Metab Pharmacokinet. 2020 Feb;45(1):1-14. doi: 10.1007/s13318-019-00570-y. Eur J Drug Metab Pharmacokinet. 2020. PMID: 31385198 Review.
-
Immuno-modulator metallo-Peptide reduces inflammatory state in obese zucker fa/fa rats.Int J Biomed Sci. 2014 Sep;10(3):172-81. Int J Biomed Sci. 2014. PMID: 25324698 Free PMC article.
-
DPP-4 inhibition improves early mortality, β cell function, and adipose tissue inflammation in db/db mice fed a diet containing sucrose and linoleic acid.Diabetol Metab Syndr. 2016 Mar 1;8:16. doi: 10.1186/s13098-016-0138-4. eCollection 2016. Diabetol Metab Syndr. 2016. PMID: 26937254 Free PMC article.
-
Therapeutic Options Targeting Oxidative Stress, Mitochondrial Dysfunction and Inflammation to Hinder the Progression of Vascular Complications of Diabetes.Front Physiol. 2019 Jan 17;9:1857. doi: 10.3389/fphys.2018.01857. eCollection 2018. Front Physiol. 2019. PMID: 30705633 Free PMC article. Review.
-
The Biological Impacts of Sitagliptin on the Pancreas of a Rat Model of Type 2 Diabetes Mellitus: Drug Interactions with Metformin.Biology (Basel). 2019 Dec 25;9(1):6. doi: 10.3390/biology9010006. Biology (Basel). 2019. PMID: 31881657 Free PMC article.
References
-
- World Health Organization. Diabetes Fact sheet N°312. Updated March 2013; http://www.who.int/mediacentre/factsheets/fs312/en/
-
- Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. (WHO/NCD/NCS/99.2). http://www.staff.ncl.ac.uk/philip.home/who_dmg.pdf.
-
- Virally M, Blicklé JF, Girard J, Halimi S, Simon D, Guillausseau PJ. Type 2 diabetes mellitus: epidemiology, pathophysiology, unmet needs and therapeutical perspectives. Diabet Med. 2007;33:231–244. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

