Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers
- PMID: 24650709
- PMCID: PMC4013273
- DOI: 10.1016/j.ydbio.2014.03.004
Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers
Abstract
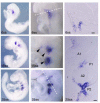
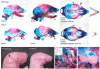
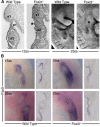
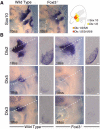
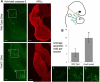
The bones of the vertebrate face develop from transient embryonic branchial arches that are populated by cranial neural crest cells. We have characterized a mouse mutant for the Forkhead family transcription factor Foxi3, which is expressed in branchial ectoderm and endoderm. Foxi3 mutant mice are not viable and display severe branchial arch-derived facial skeleton defects, including absence of all but the most distal tip of the mandible and complete absence of the inner, middle and external ear structures. Although cranial neural crest cells of Foxi3 mutants are able to migrate, populate the branchial arches, and display some elements of correct proximo-distal patterning, they succumb to apoptosis from embryonic day 9.75 onwards. We show this cell death correlates with a delay in expression of Fgf8 in branchial arch ectoderm and a failure of neural crest cells in the arches to express FGF-responsive genes. Zebrafish foxi1 is also expressed in branchial arch ectoderm and endoderm, and morpholino knock-down of foxi1 also causes apoptosis of neural crest in the branchial arches. We show that heat shock induction of fgf3 in zebrafish arch tissue can rescue cell death in foxi1 morphants. Our results suggest that Foxi3 may play a role in the establishment of signaling centers in the branchial arches that are required for neural crest survival, patterning and the subsequent development of branchial arch derivatives.
Keywords: Craniofacial development; FGF; Neural Crest; Pharyngeal arch.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129:4613–4625. - PubMed
-
- Adams DJ, Quail MA, Cox T, van der Weyden L, Gorick BD, Su Q, Chan WI, Davies R, Bonfield JK, Law F, Humphray S, Plumb B, Liu P, Rogers J, Bradley A. A genome-wide, end-sequenced 129Sv BAC library resource for targeting vector construction. Genomics. 2005;86:753–758. - PubMed
-
- Beermann F, Kaloulis K, Hofmann D, Murisier F, Bucher P, Trumpp A. Identification of evolutionarily conserved regulatory elements in the mouse Fgf8 locus. Genesis. 2006;44:1–6. - PubMed
-
- Brewer S, Jiang X, Donaldson S, Williams T, Sucov HM. Requirement for AP-2alpha in cardiac outflow tract morphogenesis. Mechanisms of development. 2002;110:139–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

