Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes
- PMID: 24650746
- PMCID: PMC4396069
- DOI: 10.1016/j.jvs.2014.01.070
Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes
Abstract
Objective: The management of arterial pathology in individuals with vascular Ehlers-Danlos syndrome (vEDS) remains a challenge. Here we describe the correlation between COL3A1 gene mutation type and the clinical phenotype in individuals with vEDS.
Methods: Individuals with confirmed molecular diagnoses of vEDS were enrolled in a multi-institutional natural history study. Data collected included demographics, clinical and family histories, arterial pathology (aneurysm, dissection, and rupture), operative details, and autopsy reports. Individuals were classified into two cohorts by the type of COL3A1 mutations and their effect on the amount of normal collagen produced: those with mutations that lead to minimal (MIN) production (10%-15%) of normal type III collagen and those with haploinsufficiency (HI) mutations that lead to production of 50% of the normal type III collagen.
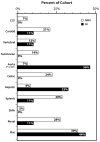
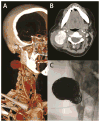
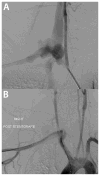
Results: A cohort of 68 individuals (72%) from 56 families had arterial pathology (44% male) with 13% HI. The HI group was older at the time of their first vascular event (mean, 42 [range, 26-58] years vs 33 [range, 8-62] years; P = .016) and had a higher incidence of aortic pathology than the MIN group (56% vs 21%; P = .025). Visceral arterial pathology was seen in 43 arteries in 23 individuals in the MIN group vs only one artery in five individuals in the HI group. Emergency surgical procedures were more likely to be undertaken when vEDS diagnosis was not known (81% vs 41%; P = .005), and 81% of these procedures were open surgical repair compared with 19% endovascular repairs (P = .019). Open and endovascular repairs were equally used in the elective setting. Postoperative complications were highest when the diagnosis of vEDS was not known (62% vs 14%; P < .001) and when procedures were undertaken in an emergency setting (5% vs 55% P < .001). Mortality due to arterial complications was 0% in the HI cohort and 21% in the MIN cohort (P = .132).
Conclusions: Arterial pathology in vEDS individuals is related to the underlying COL3A1 mutation type. The arterial pathology in individuals with HI mutations occurs at later ages with a higher incidence of aortic disease compared with other COL3A1 mutation types. Molecular diagnosis is recommended because diagnosis confirmation, appropriate surveillance, and prophylactic interventions in an elective setting improve surgical outcomes.
Copyright © 2014 Society for Vascular Surgery. All rights reserved.
Figures


Comment in
-
Discussion.J Vasc Surg. 2014 Jul;60(1):169. doi: 10.1016/j.jvs.2014.01.072. Epub 2014 Mar 18. J Vasc Surg. 2014. PMID: 24650748 No abstract available.
References
-
- Pope FM, Narcisi P, Nicholls AC, Germaine D, Pals G, Richards AJ. COL3A1 mutations cause variable clinical phenotypes including acrogeria and vascular rupture. Br J Dermatol. 1996 Aug;135(2):163–81. - PubMed
-
- Pepin MG, Byers PH. GeneReviews™ [Internet] Vol. 1993 Seattle (WA): University of Washington, Seattle; 1993–1999. Sep 02, Ehlers-Danlos Syndrome Type IV. [updated 2011 May 03]
-
- Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000 Mar 9;342(10):673–80. - PubMed
-
- Smith LT, Schwarze U, Goldstein J, Byers PH. Mutations in the COL3A1 gene result in the Ehlers-Danlos syndrome type IV and alterations in the size and distribution of the major collagen fibrils of the dermis. J Invest Dermatol. 1997 Mar;108(3):241–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

