The Kil peptide of bacteriophage λ blocks Escherichia coli cytokinesis via ZipA-dependent inhibition of FtsZ assembly
- PMID: 24651041
- PMCID: PMC3961180
- DOI: 10.1371/journal.pgen.1004217
The Kil peptide of bacteriophage λ blocks Escherichia coli cytokinesis via ZipA-dependent inhibition of FtsZ assembly
Abstract
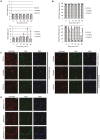
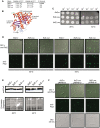
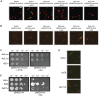
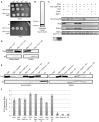
Assembly of the essential, tubulin-like FtsZ protein into a ring-shaped structure at the nascent division site determines the timing and position of cytokinesis in most bacteria and serves as a scaffold for recruitment of the cell division machinery. Here we report that expression of bacteriophage λ kil, either from a resident phage or from a plasmid, induces filamentation of Escherichia coli cells by rapid inhibition of FtsZ ring formation. Mutant alleles of ftsZ resistant to the Kil protein map to the FtsZ polymer subunit interface, stabilize FtsZ ring assembly, and confer increased resistance to endogenous FtsZ inhibitors, consistent with Kil inhibiting FtsZ assembly. Cells with the normally essential cell division gene zipA deleted (in a modified background) display normal FtsZ rings after kil expression, suggesting that ZipA is required for Kil-mediated inhibition of FtsZ rings in vivo. In support of this model, point mutations in the C-terminal FtsZ-interaction domain of ZipA abrogate Kil activity without discernibly altering FtsZ-ZipA interactions. An affinity-tagged-Kil derivative interacts with both FtsZ and ZipA, and inhibits sedimentation of FtsZ filament bundles in vitro. Together, these data inspire a model in which Kil interacts with FtsZ and ZipA in the cell to prevent FtsZ assembly into a coherent, division-competent ring structure. Phage growth assays show that kil+ phage lyse ∼30% later than kil mutant phage, suggesting that Kil delays lysis, perhaps via its interaction with FtsZ and ZipA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Weisberg RA, Gallant JA (1967) Dual function of the lambda prophage repressor. J Mol Biol 25: 537–544. - PubMed
-
- Court D, Oppenheim AB (1983) Phage lambda's accessory genes. In: Hendrix RW, Roberts JW, Stahl FW, Weisberg RA, editors. Lambda II. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. 251–278.
-
- Greer H (1975) The kil gene of bacteriophage lambda. Virology 66: 589–604. - PubMed
-
- Daniels D, Schroeder J, Szybalski W, Sanger F, Coulson A, et al... (1983) Complete annotated lambda sequence. In: Hendrix RW, Roberts JW, Stahl FW, Weisberg RA, editors. Lambda II. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. 519–676.
-
- Bahl H, Echols H, Straus DB, Court D, Crowl R, et al. (1987) Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda CIII protein. Genes Dev 1: 57–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

