Dual-layered nanogel-coated hollow lipid/polypeptide conjugate assemblies for potential pH-triggered intracellular drug release
- PMID: 24651156
- PMCID: PMC3961315
- DOI: 10.1371/journal.pone.0092268
Dual-layered nanogel-coated hollow lipid/polypeptide conjugate assemblies for potential pH-triggered intracellular drug release
Abstract
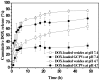
To achieve effective intracellular anticancer drug delivery, the polymeric vesicles supplemented with the pH-responsive outlayered gels as a delivery system of doxorubicin (DOX) were developed from self-assembly of the lipid/polypeptide adduct, distearin grafted poly(γ-glutamic acid) (poly(γ-GA)), followed by sequential deposition of chitosan and poly(γ-GA-co-γ-glutamyl oxysuccinimide)-g-monomethoxy poly(ethylene glycol) in combination with in situ covalent cross-linking on assembly surfaces. The resultant gel-caged polymeric vesicles (GCPVs) showed superior performance in regulating drug release in response to the external pH change. Under typical physiological conditions (pH 7.4 and 37 °C) at which the γ-GA/DOX ionic pairings remained mostly undisturbed, the dense outlayered gels of GCPVs significantly reduced the premature leakage of the uncomplexed payload. With the environmental pH being reduced from pH 7.4 to 4.7, the drug liberation was appreciably promoted by the massive disruption of the ionic γ-GA/DOX complexes along with the significant swelling of nanogel layers upon the increased protonation of chitosan chain segments. After being internalized by HeLa cells via endocytosis, GCPVs exhibited cytotoxic effect comparable to free DOX achieved by rapidly releasing the payload in intracellular acidic endosomes and lysosomes. This strongly implies the great promise of such unique GCPVs as an intracellular drug delivery carrier for potential anticancer treatment.
Conflict of interest statement
Figures








References
-
- Samad A, Sultana Y, Aqil M (2007) Liposomal drug delivery systems: an update review. Curr Drug Deliv 4: 297–305. - PubMed
-
- Elbayoumi TA, Torchilin VP (2010) Current trends in liposome research. Methods Mol Biol 605: 1–27. - PubMed
-
- Park JH, Lee S, Kim JH, Park K, Kim K, et al. (2008) Polymeric nanomedicine for cancer therapy. Prog Polym Sci 33: 113–137.
-
- Miyata K, Christie RJ, Kataoka K (2011) Polymeric micelles for nano-scale drug delivery. React Funct Polym 71: 227–234.
-
- Broz P, Driamov S, Ziegler J, Ben-Haim N, Marsch S, et al. (2006) Toward intelligent nanosize bioreactors: a pH-switchable, channel-equipped, functional polymer nanocontainer. Nano Lett 6: 2349–2353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

