Plasmodium falciparum erythrocyte invasion: combining function with immune evasion
- PMID: 24651270
- PMCID: PMC3961354
- DOI: 10.1371/journal.ppat.1003943
Plasmodium falciparum erythrocyte invasion: combining function with immune evasion
Abstract
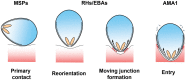
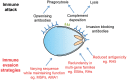
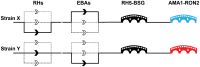
All the symptoms and pathology of malaria are caused by the intraerythrocytic stages of the Plasmodium parasite life cycle. Because Plasmodium parasites cannot replicate outside a host cell, their ability to recognize and invade erythrocytes is an essential step for both parasite survival and malaria pathogenesis. This makes invasion a conceptually attractive vaccine target, especially because it is one of the few stages when the parasite is directly exposed to the host humoral immune system. This apparent vulnerability, however, has been countered by the parasite, which has evolved sophisticated molecular mechanisms to evade the host immune response so that parasites asymptomatically replicate within immune individuals. These mechanisms include the expansion of parasite invasion ligands, resulting in multiple and apparently redundant invasion "pathways", highly polymorphic parasite surface proteins that are immunologically distinct, and parasite proteins which are poorly immunogenic. These formidable defences have so far thwarted attempts to develop an effective blood-stage vaccine, leading many to question whether there really is an exploitable chink in the parasite's immune evasion defences. Here, we review recent advances in the molecular understanding of the P. falciparum erythrocyte invasion field, discuss some of the challenges that have so far prevented the development of blood-stage vaccines, and conclude that the parasite invasion ligand RH5 represents an essential pinch point that might be vulnerable to vaccination.
Conflict of interest statement
The authors have a patent application related to the utility of
Figures
References
-
- Dvorak JA, Miller LH, Whitehouse WC, Shiroishi T (1975) Invasion of erythrocytes by malaria merozoites. Science 187: 748–750. - PubMed
-
- Hanssen E, Dekiwadia C, Riglar DT, Rug M, Lemgruber L, et al. (2013) Electron tomography of Plasmodium falciparum merozoites reveals core cellular events that underpin erythrocyte invasion. Cell Microbiol 15: 1457–1472. - PubMed
-
- Riglar DT, Richard D, Wilson DW, Boyle MJ, Dekiwadia C, et al. (2011) Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9: 9–20. - PubMed
-
- Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE (2010) Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLOS Pathog 6: e1000746 doi:10.1371/journal.ppat.1000746 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

