Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells
- PMID: 24651440
- PMCID: PMC3973226
- DOI: 10.1038/cddis.2014.66
Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells
Abstract
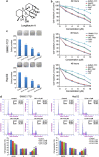
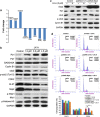
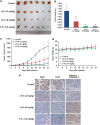
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, and is also highly resistant to conventional chemotherapy treatments. In this study, we report that Longikaurin A (LK-A), an ent-kaurane diterpenoid isolated from the plant Isodon ternifolius, induced cell cycle arrest and apoptosis in human HCC cell lines. LK-A also suppressed tumor growth in SMMC-7721 xenograft models, without inducing any notable major organ-related toxicity. LK-A treatment led to reduced expression of the proto-oncogene S phase kinase-associated protein 2 (Skp2) in SMMC-7721 cells. Lower Skp2 levels correlated with increased expression of p21 and p-cdc2 (Try15), and a corresponding decrease in protein levels of Cyclin B1 and cdc2. Overexpression of Skp2 significantly inhibited LK-A-induced cell cycle arrest in SMMC-7721 cells, suggesting that LK-A may target Skp2 to arrest cells at the G2/M phase. LK-A also induced reactive oxygen species (ROS) production and apoptosis in SMMC-7721 cells. LK-A induced phosphorylation of c-Jun N-terminal kinase (JNK), but not extracellular signal-regulated kinase and P38 MAP kinase. Treatment with, the JNK inhibitor SP600125 prevented LK-A-induced apoptosis in SMMC-7721 cells. Moreover, the antioxidant N-acetylcysteine prevented phosphorylation of both JNK and c-Jun. Taken together, these data indicate that LK-A induces cell cycle arrest and apoptosis in cancer cells by dampening Skp2 expression, and thereby activating the ROS/JNK/c-Jun signaling pathways. LK-A is therefore a potential lead compound for development of antitumor drugs targeting HCC.
Figures



References
-
- Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. - PubMed
-
- Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, et al. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25:931–938. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

