Heme oxygenase-1 derived carbon monoxide permits maturation of myeloid cells
- PMID: 24651442
- PMCID: PMC3973235
- DOI: 10.1038/cddis.2014.97
Heme oxygenase-1 derived carbon monoxide permits maturation of myeloid cells
Abstract
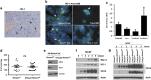
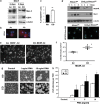
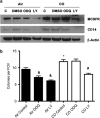
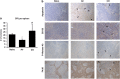
Critical functions of the immune system are maintained by the ability of myeloid progenitors to differentiate and mature into macrophages. We hypothesized that the cytoprotective gas molecule carbon monoxide (CO), generated endogenously by heme oxygenases (HO), promotes differentiation of progenitors into functional macrophages. Deletion of HO-1, specifically in the myeloid lineage (Lyz-Cre:Hmox1(flfl)), attenuated the ability of myeloid progenitors to differentiate toward macrophages and decreased the expression of macrophage markers, CD14 and macrophage colony-stimulating factor receptor (MCSFR). We showed that HO-1 and CO induced CD14 expression and efficiently increased expansion and differentiation of myeloid cells into macrophages. Further, CO sensitized myeloid cells to treatment with MCSF at low doses by increasing MCSFR expression, mediated partially through a PI3K-Akt-dependent mechanism. Exposure of mice to CO in a model of marginal bone marrow transplantation significantly improved donor myeloid cell engraftment efficiency, expansion and differentiation, which corresponded to increased serum levels of GM-CSF, IL-1α and MCP-1. Collectively, we conclude that HO-1 and CO in part are critical for myeloid cell differentiation. CO may prove to be a novel therapeutic agent to improve functional recovery of bone marrow cells in patients undergoing irradiation, chemotherapy and/or bone marrow transplantation.
Figures


References
-
- Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

