Clinical course of patients with Fabry disease who were switched from agalsidase-β to agalsidase-α
- PMID: 24651606
- PMCID: PMC4189383
- DOI: 10.1038/gim.2014.28
Clinical course of patients with Fabry disease who were switched from agalsidase-β to agalsidase-α
Abstract
Background: Between 2009 and 2012, there was a worldwide shortage of agalsidase-β for the treatment of Fabry disease. Therefore, alternative treatments were needed, including switching to a different enzyme-replacement therapy.
Purpose: This is an ongoing observational study assessing the effects of switching from agalsidase-β (1.0 mg/kg every other week) to agalsidase-α (0.2 mg/kg every other week) in 11 patients with Fabry disease.
Methods: Clinical data were collected for 5 years-2 years before switching and 3 years after switching.
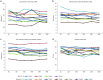
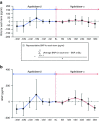
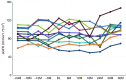
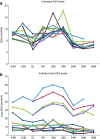
Results: Measures of renal function such as estimated glomerular filtration rate remained stable during the 3 years after switching to agalsidase-α. Improvements in cardiac mass were recorded in both male and female patients 12 months after switching to agalsidase-α, and the benefit was maintained during 36 months of follow-up. There was no significant difference in the severity of pain experienced by patients before and after switching enzyme-replacement therapy, and no difference in quality-of-life parameters. Agalsidase-α was generally well tolerated, and no patients experienced allergy or developed antibodies to agalsidase-α.
Conclusion: This observational study supports the safety of switching from agalsidase-β to agalsidase-α at the approved doses, with no loss of efficacy. It also suggests that if an infusion-related allergic reaction occurs in a patient receiving agalsidase-β, switching to agalsidase-α may be a viable option.
Figures
Similar articles
-
Agalsidase alfa: a review of its use in the management of Fabry disease.BioDrugs. 2012 Oct 1;26(5):335-54. doi: 10.2165/11209690-000000000-00000. BioDrugs. 2012. PMID: 22946754 Review.
-
Switch from agalsidase beta to agalsidase alfa in the enzyme replacement therapy of patients with Fabry disease in Latin America.Medicina (B Aires). 2017;77(3):173-179. Medicina (B Aires). 2017. PMID: 28643672 English.
-
Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal).Genet Med. 2012 Sep;14(9):779-86. doi: 10.1038/gim.2012.39. Epub 2012 Apr 12. Genet Med. 2012. PMID: 22498845 Free PMC article.
-
Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal).Genet Med. 2012 Sep;14(9):779-86. doi: 10.1038/gim.2012.81. Epub 2012 Aug 9. Genet Med. 2012. PMID: 22878505
-
Fabry disease, enzyme replacement therapy and the significance of antibody responses.J Inherit Metab Dis. 2012 Mar;35(2):227-43. doi: 10.1007/s10545-011-9400-y. Epub 2011 Oct 25. J Inherit Metab Dis. 2012. PMID: 22037707 Review.
Cited by
-
The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts.Mol Genet Metab Rep. 2019 Feb 6;19:100454. doi: 10.1016/j.ymgmr.2019.100454. eCollection 2019 Jun. Mol Genet Metab Rep. 2019. PMID: 30775256 Free PMC article. Review.
-
The Benefits of Early versus Late Therapeutic Intervention in Fabry Disease.Case Rep Genet. 2022 Dec 30;2022:3208810. doi: 10.1155/2022/3208810. eCollection 2022. Case Rep Genet. 2022. PMID: 36619006 Free PMC article.
-
A prospective 10-year study of individualized, intensified enzyme replacement therapy in advanced Fabry disease.J Inherit Metab Dis. 2015 Nov;38(6):1129-36. doi: 10.1007/s10545-015-9845-5. Epub 2015 Apr 22. J Inherit Metab Dis. 2015. PMID: 25900714 Clinical Trial.
-
Current status of the immunogenicity of enzyme replacement therapy in fabry disease.Orphanet J Rare Dis. 2025 May 26;20(1):253. doi: 10.1186/s13023-025-03705-4. Orphanet J Rare Dis. 2025. PMID: 40420145 Free PMC article. Review.
-
Agalsidase alfa and agalsidase beta in the treatment of Fabry disease: does the dose really matter?Genet Med. 2015 Jan;17(1):21-3. doi: 10.1038/gim.2014.79. Epub 2014 Jul 10. Genet Med. 2015. PMID: 25010055 Free PMC article. No abstract available.
References
-
- Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103:641–659. - PubMed
-
- Mehta A, West ML, Pintos-Morell G, et al. Therapeutic goals in the treatment of Fabry disease. Genet Med. 2010;12:713–720. - PubMed
-
- Lidove O, West ML, Pintos-Morell G, et al. Effects of enzyme replacement therapy in Fabry disease–a comprehensive review of the medical literature. Genet Med. 2010;12:668–679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

