A compact, multifunctional fusion module directs cholesterol-dependent homomultimerization and syncytiogenic efficiency of reovirus p10 FAST proteins
- PMID: 24651689
- PMCID: PMC3961370
- DOI: 10.1371/journal.ppat.1004023
A compact, multifunctional fusion module directs cholesterol-dependent homomultimerization and syncytiogenic efficiency of reovirus p10 FAST proteins
Abstract
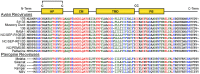
The homologous p10 fusion-associated small transmembrane (FAST) proteins of the avian (ARV) and Nelson Bay (NBV) reoviruses are the smallest known viral membrane fusion proteins, and are virulence determinants of the fusogenic reoviruses. The small size of FAST proteins is incompatible with the paradigmatic membrane fusion pathway proposed for enveloped viral fusion proteins. Understanding how these diminutive viral fusogens mediate the complex process of membrane fusion is therefore of considerable interest, from both the pathogenesis and mechanism-of-action perspectives. Using chimeric ARV/NBV p10 constructs, the 36-40-residue ectodomain was identified as the major determinant of the differing fusion efficiencies of these homologous p10 proteins. Extensive mutagenic analysis determined the ectodomain comprises two distinct, essential functional motifs. Syncytiogenesis assays, thiol-specific surface biotinylation, and liposome lipid mixing assays identified an ∼25-residue, N-terminal motif that dictates formation of a cystine loop fusion peptide in both ARV and NBV p10. Surface immunofluorescence staining, FRET analysis and cholesterol depletion/repletion studies determined the cystine loop motif is connected through a two-residue linker to a 13-residue membrane-proximal ectodomain region (MPER). The MPER constitutes a second, independent motif governing reversible, cholesterol-dependent assembly of p10 multimers in the plasma membrane. Results further indicate that: (1) ARV and NBV homomultimers segregate to distinct, cholesterol-dependent microdomains in the plasma membrane; (2) p10 homomultimerization and cholesterol-dependent microdomain localization are co-dependent; and (3) the four juxtamembrane MPER residues present in the multimerization motif dictate species-specific microdomain association and homomultimerization. The p10 ectodomain therefore constitutes a remarkably compact, multifunctional fusion module that directs syncytiogenic efficiency and species-specific assembly of p10 homomultimers into cholesterol-dependent fusion platforms in the plasma membrane.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, et al. (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 288: 1432–1435. - PubMed
-
- Halpin K, Young PL, Field HE, Mackenzie JS (2000) Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 81: 1927–1932. - PubMed
-
- Li W, Shi Z, Yu M, Ren W, Smith C, et al. (2005) Bats are natural reservoirs of SARS-like coronaviruses. Science 310: 676–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

