High-throughput epitope binning assays on label-free array-based biosensors can yield exquisite epitope discrimination that facilitates the selection of monoclonal antibodies with functional activity
- PMID: 24651868
- PMCID: PMC3961344
- DOI: 10.1371/journal.pone.0092451
High-throughput epitope binning assays on label-free array-based biosensors can yield exquisite epitope discrimination that facilitates the selection of monoclonal antibodies with functional activity
Abstract
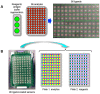
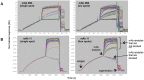
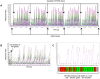
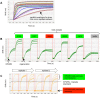
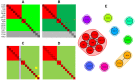
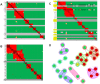
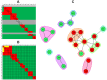
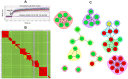
Here, we demonstrate how array-based label-free biosensors can be applied to the multiplexed interaction analysis of large panels of analyte/ligand pairs, such as the epitope binning of monoclonal antibodies (mAbs). In this application, the larger the number of mAbs that are analyzed for cross-blocking in a pairwise and combinatorial manner against their specific antigen, the higher the probability of discriminating their epitopes. Since cross-blocking of two mAbs is necessary but not sufficient for them to bind an identical epitope, high-resolution epitope binning analysis determined by high-throughput experiments can enable the identification of mAbs with similar but unique epitopes. We demonstrate that a mAb's epitope and functional activity are correlated, thereby strengthening the relevance of epitope binning data to the discovery of therapeutic mAbs. We evaluated two state-of-the-art label-free biosensors that enable the parallel analysis of 96 unique analyte/ligand interactions and nearly ten thousand total interactions per unattended run. The IBIS-MX96 is a microarray-based surface plasmon resonance imager (SPRi) integrated with continuous flow microspotting technology whereas the Octet-HTX is equipped with disposable fiber optic sensors that use biolayer interferometry (BLI) detection. We compared their throughput, versatility, ease of sample preparation, and sample consumption in the context of epitope binning assays. We conclude that the main advantages of the SPRi technology are its exceptionally low sample consumption, facile sample preparation, and unparalleled unattended throughput. In contrast, the BLI technology is highly flexible because it allows for the simultaneous interaction analysis of 96 independent analyte/ligand pairs, ad hoc sensor replacement and on-line reloading of an analyte- or ligand-array. Thus, the complementary use of these two platforms can expedite applications that are relevant to the discovery of therapeutic mAbs, depending upon the sample availability, and the number and diversity of the interactions being studied.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

