The inflammasome adaptor ASC contributes to multiple innate immune processes in the resolution of otitis media
- PMID: 24652041
- PMCID: PMC4379507
- DOI: 10.1177/1753425914526074
The inflammasome adaptor ASC contributes to multiple innate immune processes in the resolution of otitis media
Abstract
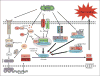
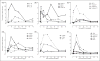
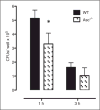
This study was designed to understand the contribution of the inflammasome and IL-1β activation in otitis media (OM). We examined the middle ear (ME) response to non-typeable Haemophilus influenzae (NTHi) in wild type (WT) mice using gene microarrays and a murine model of acute OM. Expression of members of the NOD domain-like receptor family of inflammasome genes was significantly up-regulated early in NTHi infection of the ME, potentially activating specific downstream regulatory cascades that contribute to the proliferative inflammatory response observed during OM. Expression of the pro-forms of the inflammasome targets IL-1β and IL-18 were also up-regulated. To evaluate the role of inflammasome-mediated cytokine maturation, NTHi-induced OM was examined in Asc(-/-)-deficient mice and compared with that seen in WT mice. Mice lacking the Asc gene showed near absence of IL-1β maturation in the ME and a reduction in leukocyte recruitment and infiltration to the cavity, and their macrophages exhibited reduced phagocytosis of NTHi. These inflammatory defects were linked to an increase in the degree and duration of mucosal epithelial hyperplasia in the ME of Asc(-/-) mice, as well as a delay in bacterial clearance from their MEs. These data demonstrate an important role for the inflammasome and cytokine processing in the course and resolution of OM.
Keywords: IL-1β; NTHi; cytokine; inflammation; macrophage; middle ear.
© The Author(s) 2014 Reprints and permissions: sagepub.co.uk/journalsPermissions.nav.
Figures




Similar articles
-
Panel 2- recent advance in otitis media bioinformatics.Int J Pediatr Otorhinolaryngol. 2020 Mar;130 Suppl 1(Suppl 1):109834. doi: 10.1016/j.ijporl.2019.109834. Epub 2019 Dec 18. Int J Pediatr Otorhinolaryngol. 2020. PMID: 31899006 Free PMC article. Review.
-
Otitis Media and Nasopharyngeal Colonization in ccl3-/- Mice.Infect Immun. 2017 Oct 18;85(11):e00148-17. doi: 10.1128/IAI.00148-17. Print 2017 Nov. Infect Immun. 2017. PMID: 28847849 Free PMC article.
-
Expression of surfactant Protein-A in the Haemophilus influenzae-induced otitis media in a rat model.Int J Pediatr Otorhinolaryngol. 2018 Sep;112:61-66. doi: 10.1016/j.ijporl.2018.06.030. Epub 2018 Jun 19. Int J Pediatr Otorhinolaryngol. 2018. PMID: 30055742
-
Myeloid differentiation primary response gene 88 is required for the resolution of otitis media.J Infect Dis. 2008 Dec 15;198(12):1862-9. doi: 10.1086/593213. J Infect Dis. 2008. PMID: 18986247 Free PMC article.
-
Impact of protein D-containing pneumococcal conjugate vaccines on non-typeable Haemophilus influenzae acute otitis media and carriage.Expert Rev Vaccines. 2017 Jul;16(7):1-14. doi: 10.1080/14760584.2017.1333905. Epub 2017 Jun 7. Expert Rev Vaccines. 2017. PMID: 28571504 Review.
Cited by
-
The ECRG4 cleavage product augurin binds the endotoxin receptor and influences the innate immune response during otitis media.Front Genet. 2022 Aug 26;13:932555. doi: 10.3389/fgene.2022.932555. eCollection 2022. Front Genet. 2022. PMID: 36092940 Free PMC article.
-
Resolution of otitis media in a humanized mouse model.Front Genet. 2022 Nov 9;13:958540. doi: 10.3389/fgene.2022.958540. eCollection 2022. Front Genet. 2022. PMID: 36437913 Free PMC article.
-
Lack of the hyaluronan receptor CD44 affects the course of bacterial otitis media and reduces leukocyte recruitment to the middle ear.BMC Immunol. 2019 Jun 21;20(1):20. doi: 10.1186/s12865-019-0302-3. BMC Immunol. 2019. PMID: 31226944 Free PMC article.
-
The transcriptome of a complete episode of acute otitis media.BMC Genomics. 2015 Apr 3;16(1):259. doi: 10.1186/s12864-015-1475-7. BMC Genomics. 2015. PMID: 25888408 Free PMC article.
-
Panel 2- recent advance in otitis media bioinformatics.Int J Pediatr Otorhinolaryngol. 2020 Mar;130 Suppl 1(Suppl 1):109834. doi: 10.1016/j.ijporl.2019.109834. Epub 2019 Dec 18. Int J Pediatr Otorhinolaryngol. 2020. PMID: 31899006 Free PMC article. Review.
References
-
- Baxt LA, Garza-Mayers AC, Goldberg MB. Bacterial sub-version of host innate immune pathways. Science. 2013;340:697–701. - PubMed
-
- Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: Studying an integrated system-wide organ. Nat Immunol. 2010;11:558–560. - PubMed
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. - PubMed
-
- Muruve DA, Petrilli V, Zaiss AK, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

