Hemolytic lectin CEL-III heptamerizes via a large structural transition from α-helices to a β-barrel during the transmembrane pore formation process
- PMID: 24652284
- PMCID: PMC4007468
- DOI: 10.1074/jbc.M113.541896
Hemolytic lectin CEL-III heptamerizes via a large structural transition from α-helices to a β-barrel during the transmembrane pore formation process
Abstract
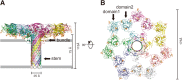
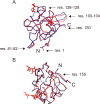
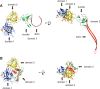
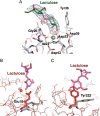
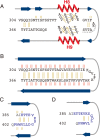
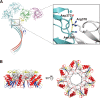
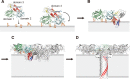
CEL-III is a hemolytic lectin isolated from the sea cucumber Cucumaria echinata. This lectin is composed of two carbohydrate-binding domains (domains 1 and 2) and one oligomerization domain (domain 3). After binding to the cell surface carbohydrate chains through domains 1 and 2, domain 3 self-associates to form transmembrane pores, leading to cell lysis or death, which resembles other pore-forming toxins of diverse organisms. To elucidate the pore formation mechanism of CEL-III, the crystal structure of the CEL-III oligomer was determined. The CEL-III oligomer has a heptameric structure with a long β-barrel as a transmembrane pore. This β-barrel is composed of 14 β-strands resulting from a large structural transition of α-helices accommodated in the interface between domains 1 and 2 and domain 3 in the monomeric structure, suggesting that the dissociation of these α-helices triggered their structural transition into a β-barrel. After heptamerization, domains 1 and 2 form a flat ring, in which all carbohydrate-binding sites remain bound to cell surface carbohydrate chains, stabilizing the transmembrane β-barrel in a position perpendicular to the plane of the lipid bilayer.
Keywords: Carbohydrate; Hemolysin; Lectin; Membrane; Oligomer; Pore-forming Toxin; Toxins; X-ray Crystallography; β-Barrel.
Figures


Similar articles
-
Galactose-Specific, Hemolytic Lectin CEL-III from Cucumaria echinata.Methods Mol Biol. 2020;2132:159-164. doi: 10.1007/978-1-0716-0430-4_16. Methods Mol Biol. 2020. PMID: 32306324
-
Crystal structure of the hemolytic lectin CEL-III isolated from the marine invertebrate Cucumaria echinata: implications of domain structure for its membrane pore-formation mechanism.J Biol Chem. 2004 Aug 27;279(35):37133-41. doi: 10.1074/jbc.M404065200. Epub 2004 Jun 11. J Biol Chem. 2004. PMID: 15194688
-
Identification of the amino acid residues involved in the hemolytic activity of the Cucumaria echinata lectin CEL-III.Biochim Biophys Acta. 2013 Aug;1830(8):4211-7. doi: 10.1016/j.bbagen.2013.04.010. Epub 2013 Apr 11. Biochim Biophys Acta. 2013. PMID: 23583369
-
Ion channels and bacterial infection: the case of beta-barrel pore-forming protein toxins of Staphylococcus aureus.FEBS Lett. 2003 Sep 18;552(1):54-60. doi: 10.1016/s0014-5793(03)00850-0. FEBS Lett. 2003. PMID: 12972152 Review.
-
Beta-barrel membrane protein folding and structure viewed through the lens of alpha-hemolysin.Biochim Biophys Acta. 2003 Jan 10;1609(1):19-27. doi: 10.1016/s0005-2736(02)00663-6. Biochim Biophys Acta. 2003. PMID: 12507754 Review.
Cited by
-
The choanoflagellate pore-forming lectin SaroL-1 punches holes in cancer cells by targeting the tumor-related glycosphingolipid Gb3.Commun Biol. 2022 Sep 12;5(1):954. doi: 10.1038/s42003-022-03869-w. Commun Biol. 2022. PMID: 36097056 Free PMC article.
-
Identification, Characterization, and X-ray Crystallographic Analysis of a Novel Type of Lectin AJLec from the Sea Anemone Anthopleura japonica.Sci Rep. 2018 Aug 1;8(1):11516. doi: 10.1038/s41598-018-29498-0. Sci Rep. 2018. PMID: 30068923 Free PMC article.
-
Conformational changes during pore formation by the perforin-related protein pleurotolysin.PLoS Biol. 2015 Feb 5;13(2):e1002049. doi: 10.1371/journal.pbio.1002049. eCollection 2015 Feb. PLoS Biol. 2015. PMID: 25654333 Free PMC article.
-
Tandem-repeat lectins: structural and functional insights.Glycobiology. 2024 May 26;34(7):cwae041. doi: 10.1093/glycob/cwae041. Glycobiology. 2024. PMID: 38857376 Free PMC article.
-
Pore-forming toxins: ancient, but never really out of fashion.Nat Rev Microbiol. 2016 Feb;14(2):77-92. doi: 10.1038/nrmicro.2015.3. Epub 2015 Dec 7. Nat Rev Microbiol. 2016. PMID: 26639780 Review.
References
-
- Song L., Hobaugh M. R., Shustak C., Cheley S., Bayley H., Gouaux J. E. (1996) Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1866 - PubMed
-
- Yamashita K., Kawai Y., Tanaka Y., Hirano N., Kaneko J., Tomita N., Ohta M., Kamio Y., Yao M., Tanaka I. (2011) Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components. Proc. Natl. Acad. Sci. U.S.A. 108, 17314–17319 - PMC - PubMed
-
- Voskoboinik I., Smyth M. J., Trapani J. A. (2006) Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 6, 940–952 - PubMed
-
- Mancheño J. M., Tateno H., Goldstein I. J., Martínez-Ripoll M., Hermoso J. A. (2005) Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J. Biol. Chem. 280, 17251–17259 - PubMed
-
- Hatakeyama T., Kohzaki H., Nagatomo H., Yamasaki N. (1994) Purification and characterization of four Ca2+-dependent lectins from the marine invertebrate, Cucumaria echinata. J. Biochem. 116, 209–214 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

