Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth
- PMID: 24652285
- PMCID: PMC4036339
- DOI: 10.1074/jbc.M114.550541
Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth
Abstract
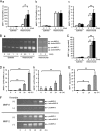
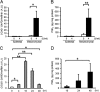
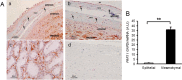
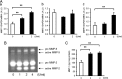
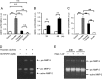
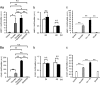
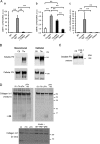
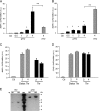
Here, we investigated the effects of thrombin on matrix metalloproteinases (MMPs) and prostaglandin (PG) synthesis in fetal membranes. Thrombin activity was increased in human amnion from preterm deliveries. Treatment of mesenchymal, but not epithelial, cells with thrombin resulted in increased MMP-1 and MMP-9 mRNA and enzymatic activity. Thrombin also increased COX2 mRNA and PGE2 in these cells. Protease-activated receptor-1 (PAR-1) was localized to amnion mesenchymal and decidual cells. PAR-1-specific inhibitors and activating peptides indicated that thrombin-induced up-regulation of MMP-9 was mediated via PAR-1. In contrast, thrombin-induced up-regulation of MMP-1 and COX-2 was mediated through Toll-like receptor-4, possibly through thrombin-induced release of soluble fetal fibronectin. In vivo, thrombin-injected pregnant mice delivered preterm. Mmp8, Mmp9, and Mmp13, and PGE2 content was increased significantly in fetal membranes from thrombin-injected animals. These results indicate that thrombin acts through multiple mechanisms to activate MMPs and PGE2 synthesis in amnion.
Keywords: Amnion; Cyclooxygenase (COX) Pathway; Decidua; Fetal Membrane; Fibronectin; Matrix Metalloproteinase (MMP); Prostaglandins; Protease-activated Receptor-1; Thrombin.
Figures

References
-
- Chaiworapongsa T., Espinoza J., Yoshimatsu J., Kim Y. M., Bujold E., Edwin S., Yoon B. H., Romero R. (2002) Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J. Matern. Fetal Neonatal Med. 11, 368–373 - PubMed
-
- Erez O., Romer R., Vaisbuch E., Chaiworapongsa T., Kusanovic J. P., Mazaki-Tovi S., Gotsch F., Gomez R., Maymon E., Pacora P., Edwin S. S., Kim C. J., Than N. G., Mittal P., Yeo L., Dong Z., Yoon B. H., Hassan S. S., Mazor M. (2009) Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J. Matern. Fetal Neonatal Med. 22, 971–982 - PMC - PubMed
-
- Rosen T., Kuczynski E., O'Neill L. M., Funai E. F., Lockwood C. J. (2001) Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J. Matern Fetal Med. 10, 297–300 - PubMed
-
- Nagy S., Bush M., Stone J., Lapinski R. H., Gardó S. (2003) Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet. Gynecol. 102, 94–100 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

