Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib
- PMID: 24652387
- PMCID: PMC3983809
- DOI: 10.1634/theoncologist.2013-0114
Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib
Abstract
Introduction: Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1), may underestimate activity and does not predict survival in patients with hepatocellular carcinoma (HCC) treated with sorafenib. This study assessed the value of alternative radiological criteria to evaluate response in HCC patients treated with sorafenib.
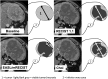
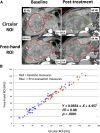
Patients and methods: A retrospective blinded central analysis was performed of computed tomography (CT) scans from baseline and the first tumor evaluation in consecutive patients treated with sorafenib over a 2-year period in a single institution. Four different evaluation criteria were used: Choi, European Association for the Study of the Liver (EASL), modified RECIST (mRECIST), and RECIST 1.1.
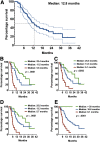
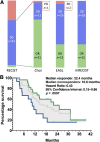
Results: Among 82 HCC patients, 64 with Barcelona Clinic Liver Cancer stage B-C were evaluable with a median follow-up of 22 months. Median duration of sorafenib treatment was 5.7 months, and median overall survival was 12.8 months. At the time of the first CT scan, performed after a median of 2.1 months, Choi, EASL, mRECIST, and RECIST 1.1 identified 51%, 28%, 28%, and 3% objective responses, respectively. Responders by all criteria showed consistent overall survival >20 months. Among patients with stable disease according to RECIST 1.1, those identified as responders by Choi had significantly better overall survival than Choi nonresponders (22.4 vs. 10.6 months; hazard ratio: 0.43, 95% confidence interval: 0.15-0.86, p = .0097).
Conclusion: Choi, EASL, and mRECIST criteria appear more appropriate than RECIST 1.1 to identify responders with long survival among advanced HCC patients benefiting from sorafenib.
Keywords: Antiangiogenic agents; Computed tomography; Density; Targeted therapy; Tumor evaluation.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
-
- World Health Organization. Geneva, Switzerland: World Health Organization; 1979. WHO handbook for reporting results of cancer treatment.
-
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

