Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains
- PMID: 24652590
- PMCID: PMC4093953
- DOI: 10.1002/pro.2460
Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains
Abstract
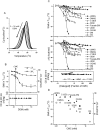
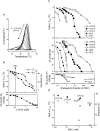
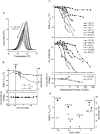
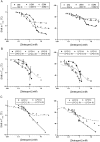
Detergent interaction with extramembranous soluble domains (ESDs) is not commonly considered an important determinant of integral membrane protein (IMP) behavior during purification and crystallization, even though ESDs contribute to the stability of many IMPs. Here we demonstrate that some generally nondenaturing detergents critically destabilize a model ESD, the first nucleotide-binding domain (NBD1) from the human cystic fibrosis transmembrane conductance regulator (CFTR), a model IMP. Notably, the detergents show equivalent trends in their influence on the stability of isolated NBD1 and full-length CFTR. We used differential scanning calorimetry (DSC) and circular dichroism (CD) spectroscopy to monitor changes in NBD1 stability and secondary structure, respectively, during titration with a series of detergents. Their effective harshness in these assays mirrors that widely accepted for their interaction with IMPs, i.e., anionic > zwitterionic > nonionic. It is noteworthy that including lipids or nonionic detergents is shown to mitigate detergent harshness, as will limiting contact time. We infer three thermodynamic mechanisms from the observed thermal destabilization by monomer or micelle: (i) binding to the unfolded state with no change in the native structure (all detergent classes); (ii) native state binding that alters thermodynamic properties and perhaps conformation (nonionic detergents); and (iii) detergent binding that directly leads to denaturation of the native state (anionic and zwitterionic). These results demonstrate that the accepted model for the harshness of detergents applies to their interaction with an ESD. It is concluded that destabilization of extramembranous soluble domains by specific detergents will influence the stability of some IMPs during purification.
Keywords: CD; CFTR; DSC; NBD1; detergent interaction; extramembrane domain; membrane protein; soluble domain; thermal unfolding.
© 2014 The Protein Society.
Figures


Similar articles
-
A Guide to Differential Scanning Calorimetry of Membrane and Soluble Proteins in Detergents.Methods Enzymol. 2016;567:319-58. doi: 10.1016/bs.mie.2015.08.014. Epub 2015 Oct 20. Methods Enzymol. 2016. PMID: 26794360
-
Thermodynamic characterization of the exchange of detergents and amphipols at the surfaces of integral membrane proteins.Langmuir. 2009 Nov 3;25(21):12623-34. doi: 10.1021/la9018772. Langmuir. 2009. PMID: 19594168
-
Lipid-like Peptides can Stabilize Integral Membrane Proteins for Biophysical and Structural Studies.Chembiochem. 2017 Sep 5;18(17):1735-1742. doi: 10.1002/cbic.201700235. Epub 2017 Jul 17. Chembiochem. 2017. PMID: 28603929 Free PMC article.
-
Folding and stability of integral membrane proteins in amphipols.Arch Biochem Biophys. 2014 Dec 15;564:327-43. doi: 10.1016/j.abb.2014.10.013. Epub 2014 Oct 30. Arch Biochem Biophys. 2014. PMID: 25449655 Review.
-
Membrane proteins, detergents and crystals: what is the state of the art?Acta Crystallogr F Struct Biol Commun. 2014 Dec 1;70(Pt 12):1576-83. doi: 10.1107/S2053230X14025035. Epub 2014 Nov 28. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25484203 Free PMC article. Review.
Cited by
-
Conformationally flexible core-bearing detergents with a hydrophobic or hydrophilic pendant: Effect of pendant polarity on detergent conformation and membrane protein stability.Acta Biomater. 2021 Jul 1;128:393-407. doi: 10.1016/j.actbio.2021.04.043. Epub 2021 Apr 29. Acta Biomater. 2021. PMID: 33933694 Free PMC article.
-
Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure.Proc Natl Acad Sci U S A. 2016 Aug 23;113(34):9509-14. doi: 10.1073/pnas.1610403113. Epub 2016 Aug 8. Proc Natl Acad Sci U S A. 2016. PMID: 27503893 Free PMC article.
-
A Class of Rigid Linker-bearing Glucosides for Membrane Protein Structural Study.Chem Sci. 2016 Mar 1;7(3):1933-1939. doi: 10.1039/C5SC02900G. Epub 2015 Dec 16. Chem Sci. 2016. PMID: 27110345 Free PMC article.
-
Conformationally Restricted Monosaccharide-Cored Glycoside Amphiphiles: The Effect of Detergent Headgroup Variation on Membrane Protein Stability.ACS Chem Biol. 2019 Aug 16;14(8):1717-1726. doi: 10.1021/acschembio.9b00166. Epub 2019 Jul 29. ACS Chem Biol. 2019. PMID: 31305987 Free PMC article.
-
Identification of binding sites for ivacaftor on the cystic fibrosis transmembrane conductance regulator.iScience. 2021 May 15;24(6):102542. doi: 10.1016/j.isci.2021.102542. eCollection 2021 Jun 25. iScience. 2021. PMID: 34142049 Free PMC article.
References
-
- Wiener MC. A pedestrian guide to membrane protein crystallization. Methods. 2004;34:364–372. - PubMed
-
- Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. - PubMed
-
- Garavito RM, Ferguson-Miller S. Detergents as tools in membrane biochemistry. J Biol Chem. 2001;276:32403–32406. - PubMed
-
- Prive GG. Detergents for the stabilization and crystallization of membrane proteins. Methods. 2007;41:388–397. - PubMed
-
- Linke D. Detergents: an overview. Methods Enzymol. 2009;463:63034–63042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

