Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis
- PMID: 24652604
- PMCID: PMC4000413
- DOI: 10.1007/s00280-014-2440-x
Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis
Abstract
Background: Hangeshashinto (TJ-14, a Kampo medicine), which reduces the level of prostaglandin E2 and affects the cyclooxygenase activity, alleviates chemotherapy-induced oral mucositis (COM). We conducted a randomized comparative trial to investigate whether TJ-14 prevents and controls COM in patients with gastric cancer.
Methods: We randomly assigned patients with gastric cancer who developed moderate-to-severe oral mucositis (CTCAE v4.0 grade ≧1) during any cycle of chemotherapy to receive either TJ-14 or a placebo as a double-blind trial. The patients received a placebo or TJ-14 for 2-6 weeks according to the chemotherapy regimen from the beginning of the next course of chemotherapy. The primary end point was the incidence of grade ≧2 oral mucositis in the protocol treatment course, and the secondary end points were the time to disappearance of oral mucositis and the incidence of adverse events.
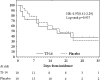
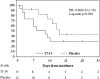
Results: Following the key opening of the blinding protocol, we analyzed 91 eligible patients (TJ-14: 45, placebo: 46) using a "per protocol set" analysis. The incidence of ≧grade 2 COM was 40.0 % in the TJ-14 group and 41.3 % in the placebo group (p = 0.588). The median duration of ≧grade 2 COM was 14 days in the TJ-14 group and 16 days in the placebo group (p = 0.894). Meanwhile, the median duration of any grade of COM was 9 days in the TJ-14 group and 17 days in the placebo group among the patients who developed grade 1 symptoms during the screening cycle [hazard ratio 0.60; 95 % CI (0.23-1.59), p = 0.290].
Conclusions: Although TJ-14 treatment did not reduce the incidence of ≥2 COM in the patients who developed mucositis during chemotherapy for gastric cancer, a trend was observed in which TJ-14 reduced the risk of COM in the patients who developed grade 1 COM during the screening cycle. Further, phase III studies with a larger sample size are needed to clarify the protective effects of TJ-14 for COM.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical