Trafficking to the apical and basolateral membranes in polarized epithelial cells
- PMID: 24652803
- PMCID: PMC4073435
- DOI: 10.1681/ASN.2013080883
Trafficking to the apical and basolateral membranes in polarized epithelial cells
Abstract
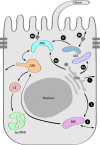
Renal epithelial cells must maintain distinct protein compositions in their apical and basolateral membranes in order to perform their transport functions. The creation of these polarized protein distributions depends on sorting signals that designate the trafficking route and site of ultimate functional residence for each protein. Segregation of newly synthesized apical and basolateral proteins into distinct carrier vesicles can occur at the trans-Golgi network, recycling endosomes, or a growing assortment of stations along the cellular trafficking pathway. The nature of the specific sorting signal and the mechanism through which it is interpreted can influence the route a protein takes through the cell. Cell type-specific variations in the targeting motifs of a protein, as are evident for Na,K-ATPase, demonstrate a remarkable capacity to adapt sorting pathways to different developmental states or physiologic requirements. This review summarizes our current understanding of apical and basolateral trafficking routes in polarized epithelial cells.
Keywords: cell and transport physiology; epithelial; renal cell biology.
Copyright © 2014 by the American Society of Nephrology.
Figures

References
-
- Futter CE, Connolly CN, Cutler DF, Hopkins CR: Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem 270: 10999–11003, 1995 - PubMed
-
- Laird V, Spiess M: A novel assay to demonstrate an intersection of the exocytic and endocytic pathways at early endosomes. Exp Cell Res 260: 340–345, 2000 - PubMed
-
- Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW: Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic 1: 124–140, 2000 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

