β-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche
- PMID: 24653033
- PMCID: PMC4096864
- DOI: 10.1126/science.1248373
β-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche
Abstract
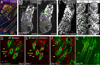
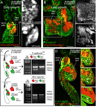
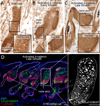
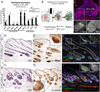
Wnt/β-catenin signaling is critical for tissue regeneration. However, it is unclear how β-catenin controls stem cell behaviors to coordinate organized growth. Using live imaging, we show that activation of β-catenin specifically within mouse hair follicle stem cells generates new hair growth through oriented cell divisions and cellular displacement. β-Catenin activation is sufficient to induce hair growth independently of mesenchymal dermal papilla niche signals normally required for hair regeneration. Wild-type cells are co-opted into new hair growths by β-catenin mutant cells, which non-cell autonomously activate Wnt signaling within the neighboring wild-type cells via Wnt ligands. This study demonstrates a mechanism by which Wnt/β-catenin signaling controls stem cell-dependent tissue growth non-cell autonomously and advances our understanding of the mechanisms that drive coordinated regeneration.
Conflict of interest statement
We declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

